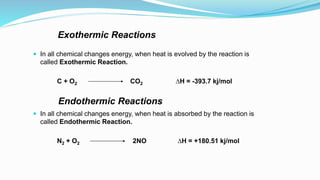
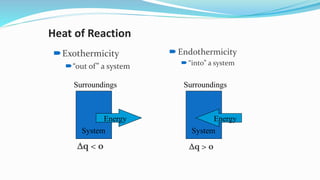
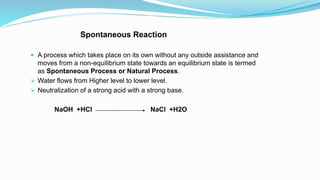
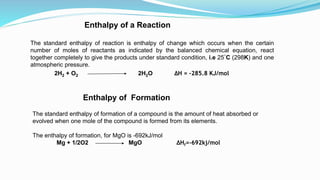
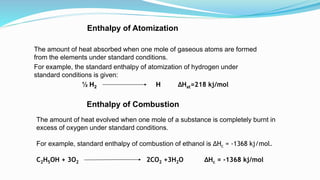
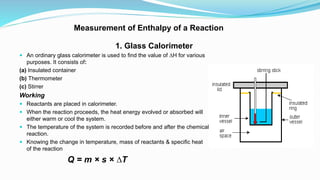
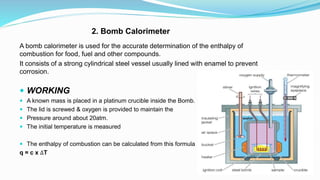
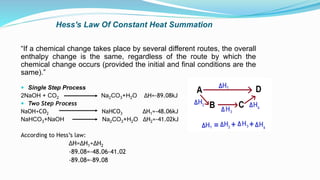
Thermochemistry is the study of heat changes in chemical reactions. Exothermic reactions evolve heat while endothermic reactions absorb heat. The enthalpy of a reaction is the quantity of heat absorbed or evolved. Standard enthalpy of reaction is measured under standard conditions. Hess's law states that the enthalpy change of a reaction is the same regardless of the reaction pathway. Thermochemistry is applied in pyrometallurgy which involves processes like calcination, roasting, smelting, and refining to extract metals from ores.