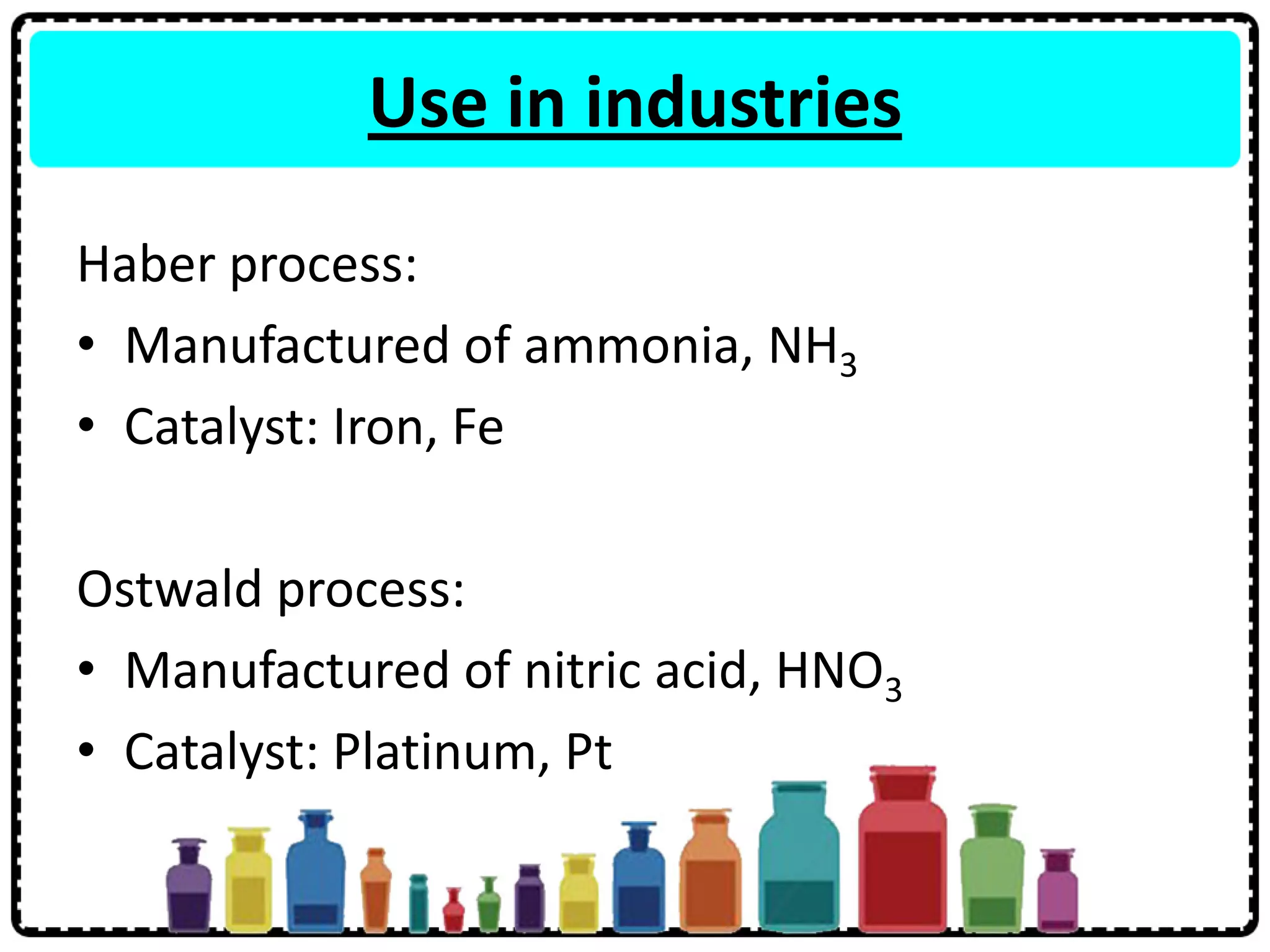
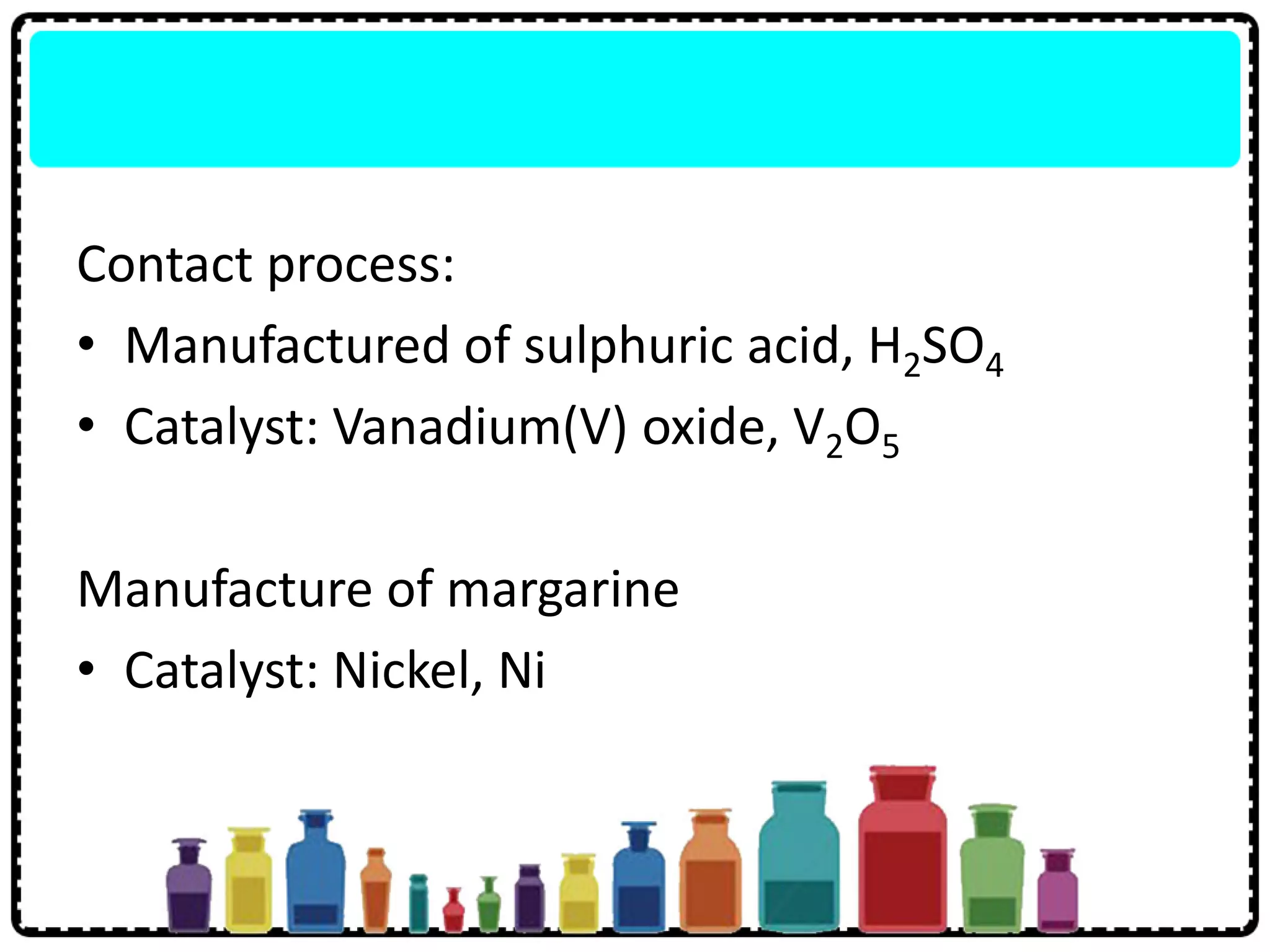
The document summarizes key aspects of the periodic table including:
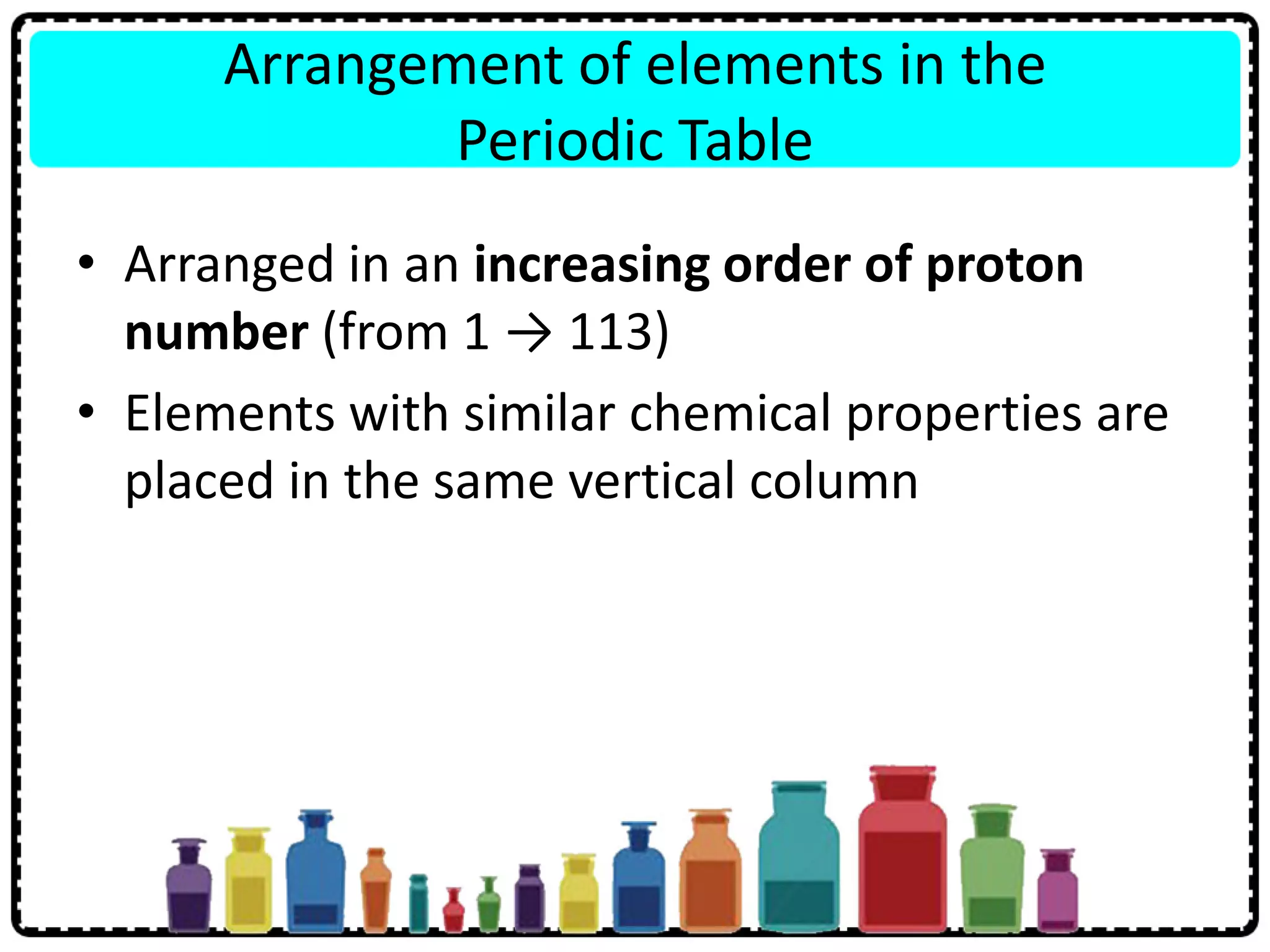
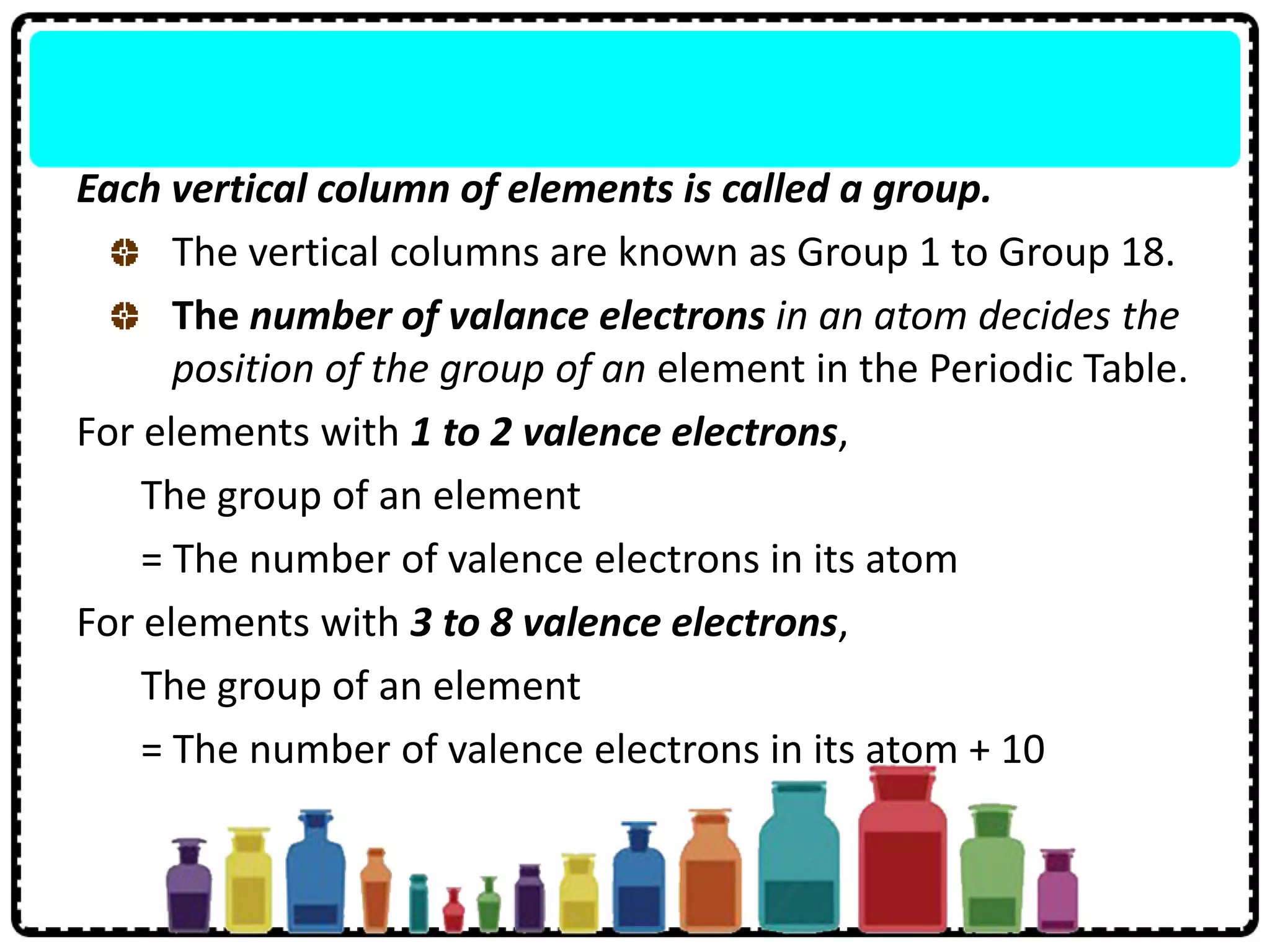
1) Elements are arranged in order of increasing proton number and elements with similar properties are in the same group, with groups 1-18 and periods 1-7.
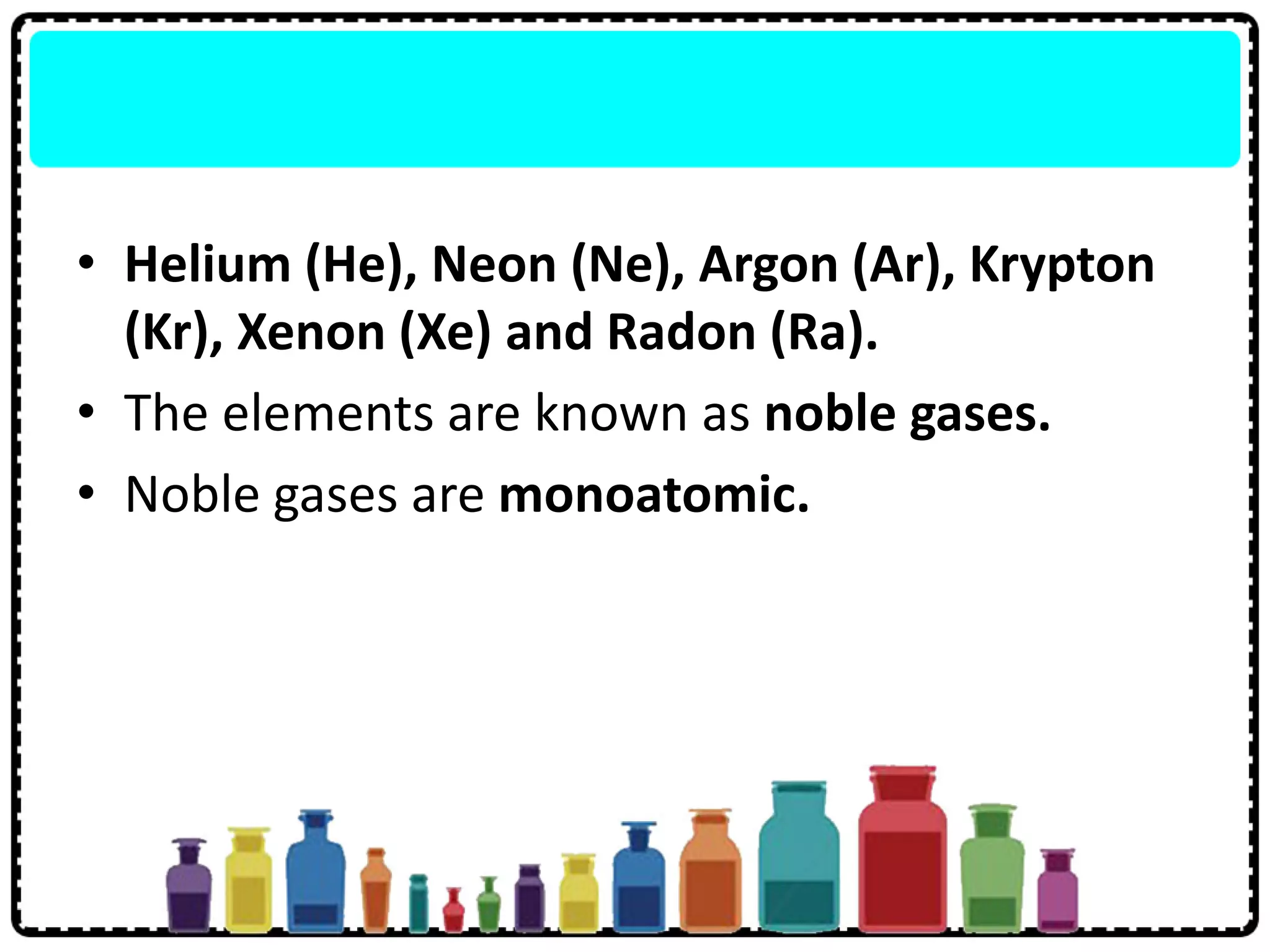
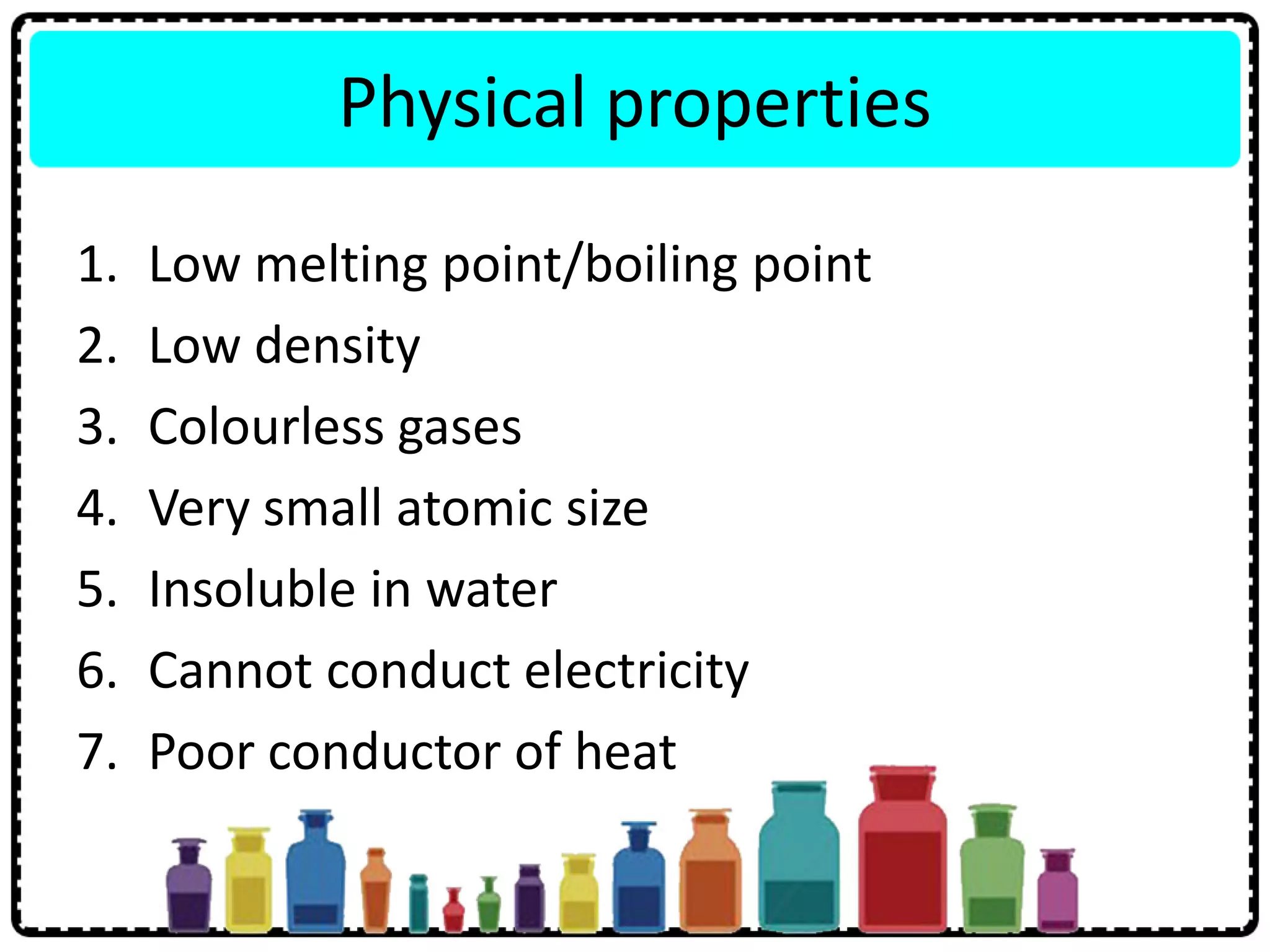
2) Group 18 elements are noble gases with full outer shells making them chemically inert monoatomic gases.
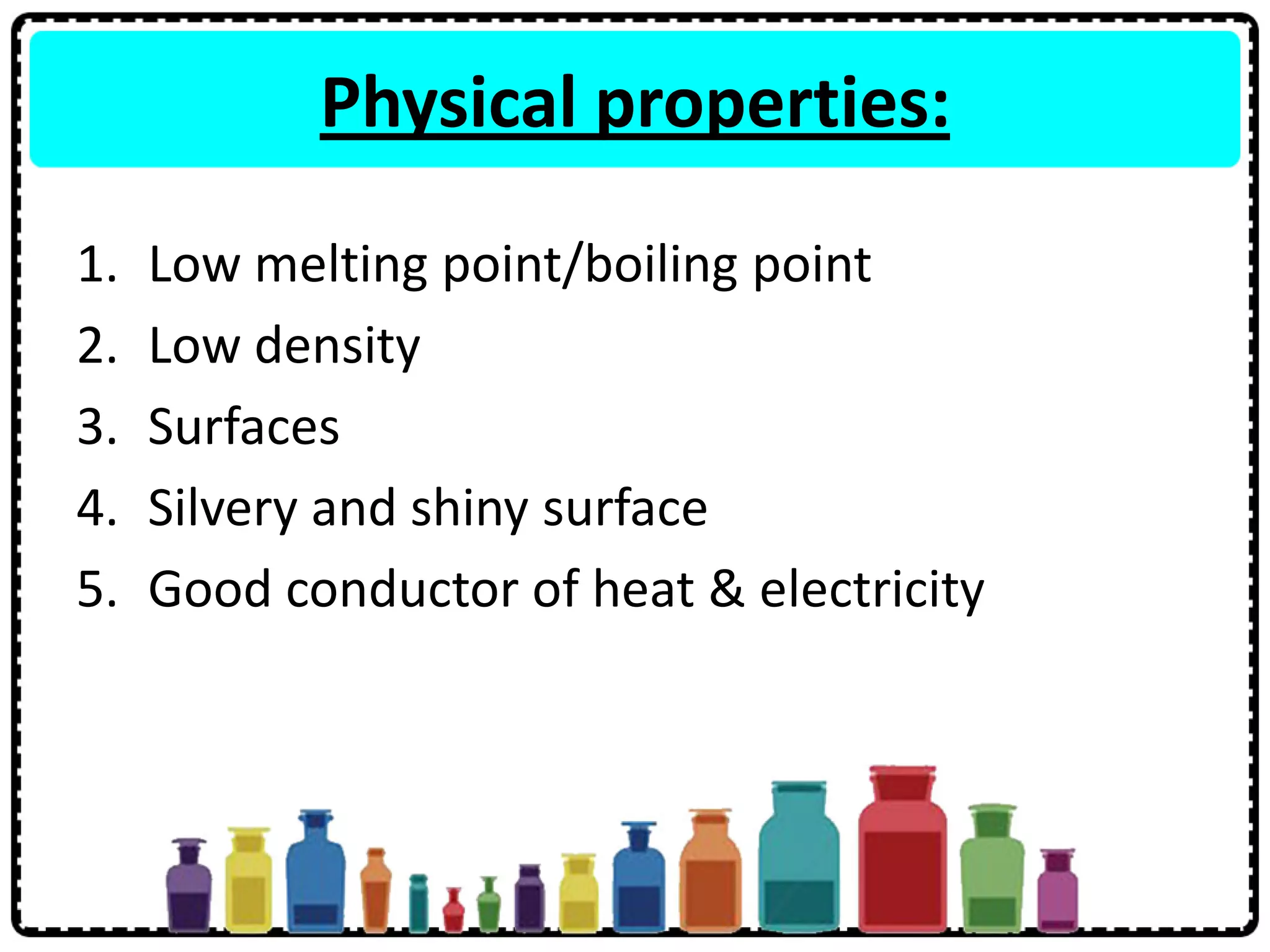
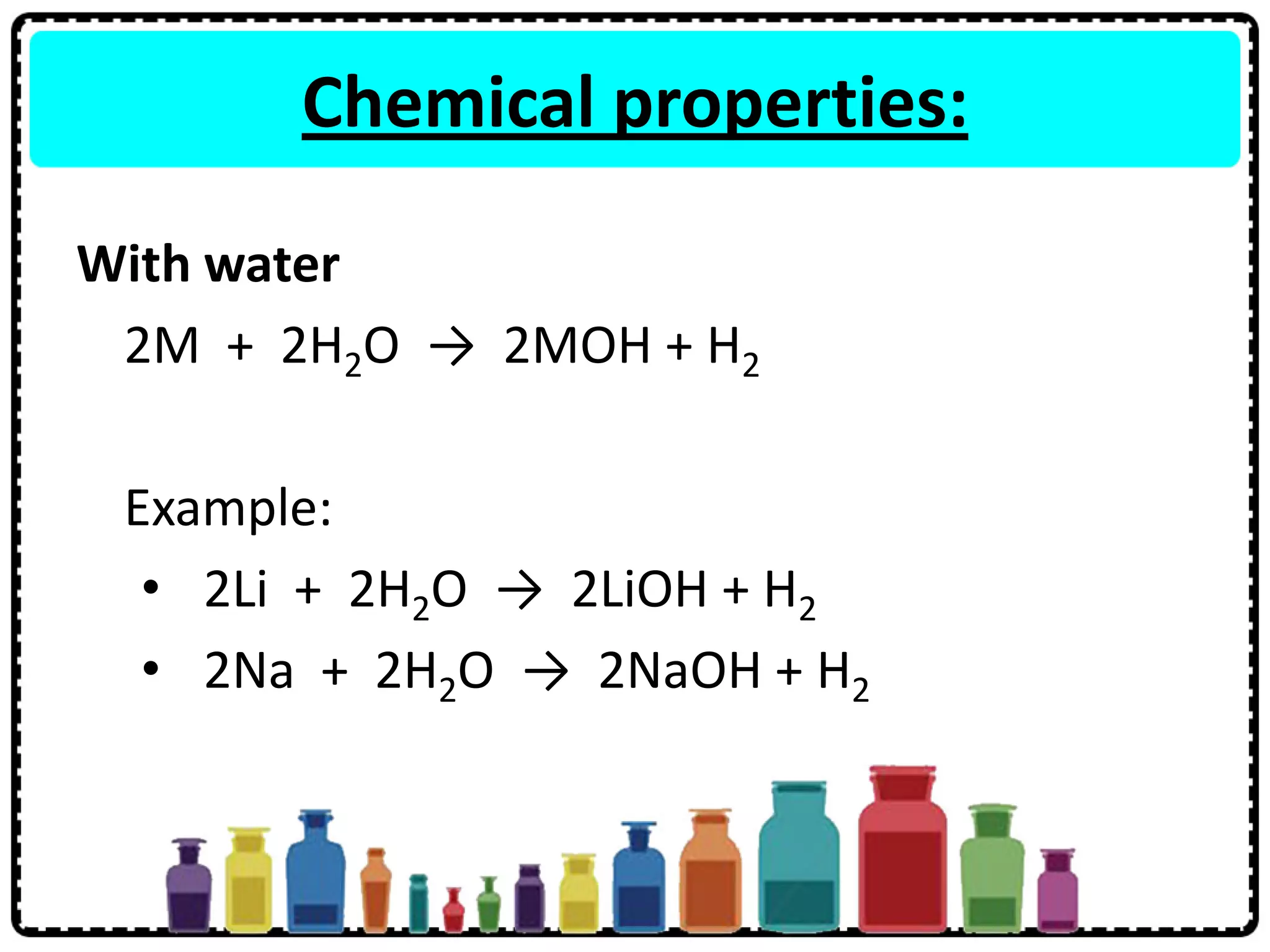
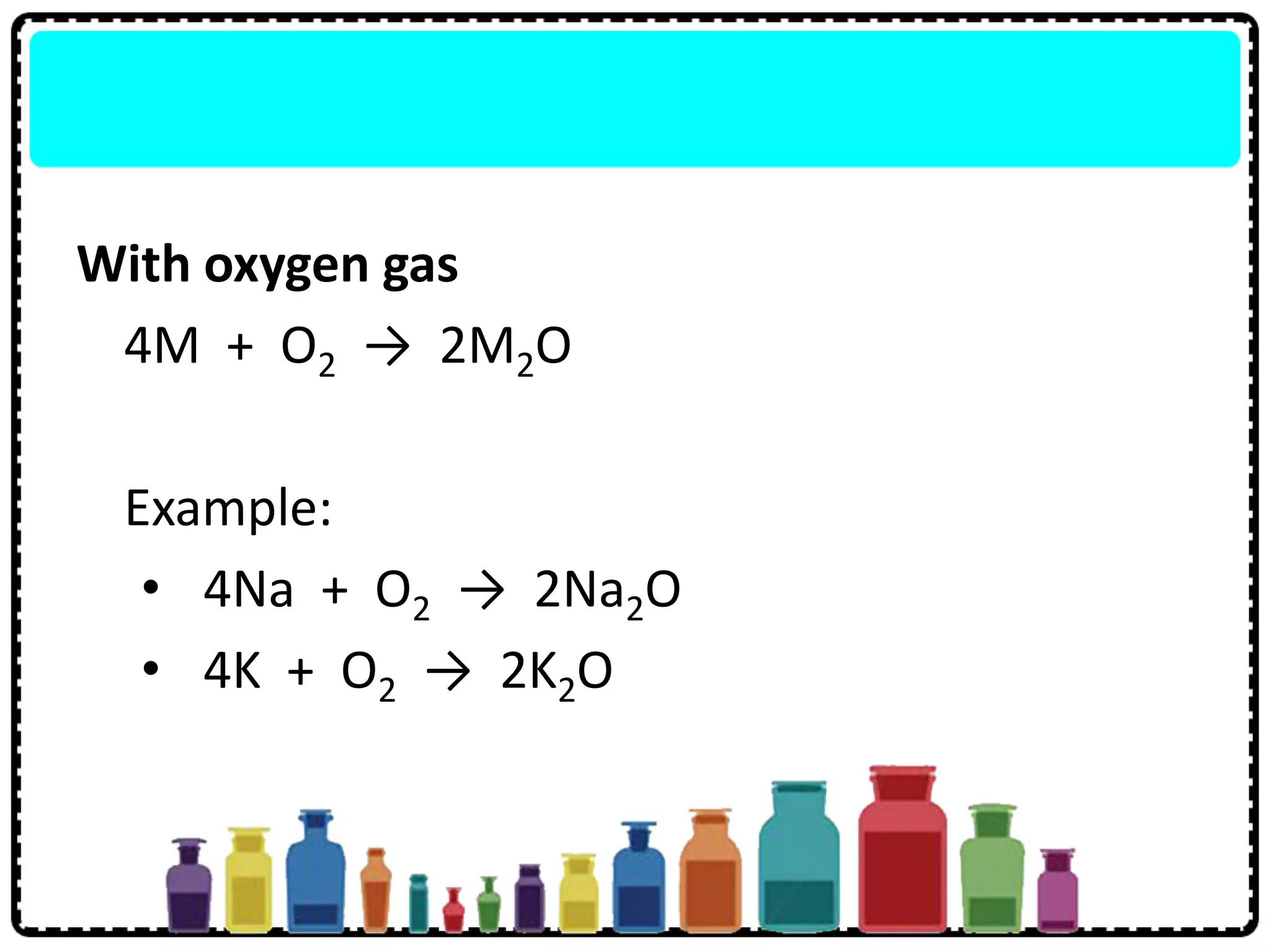
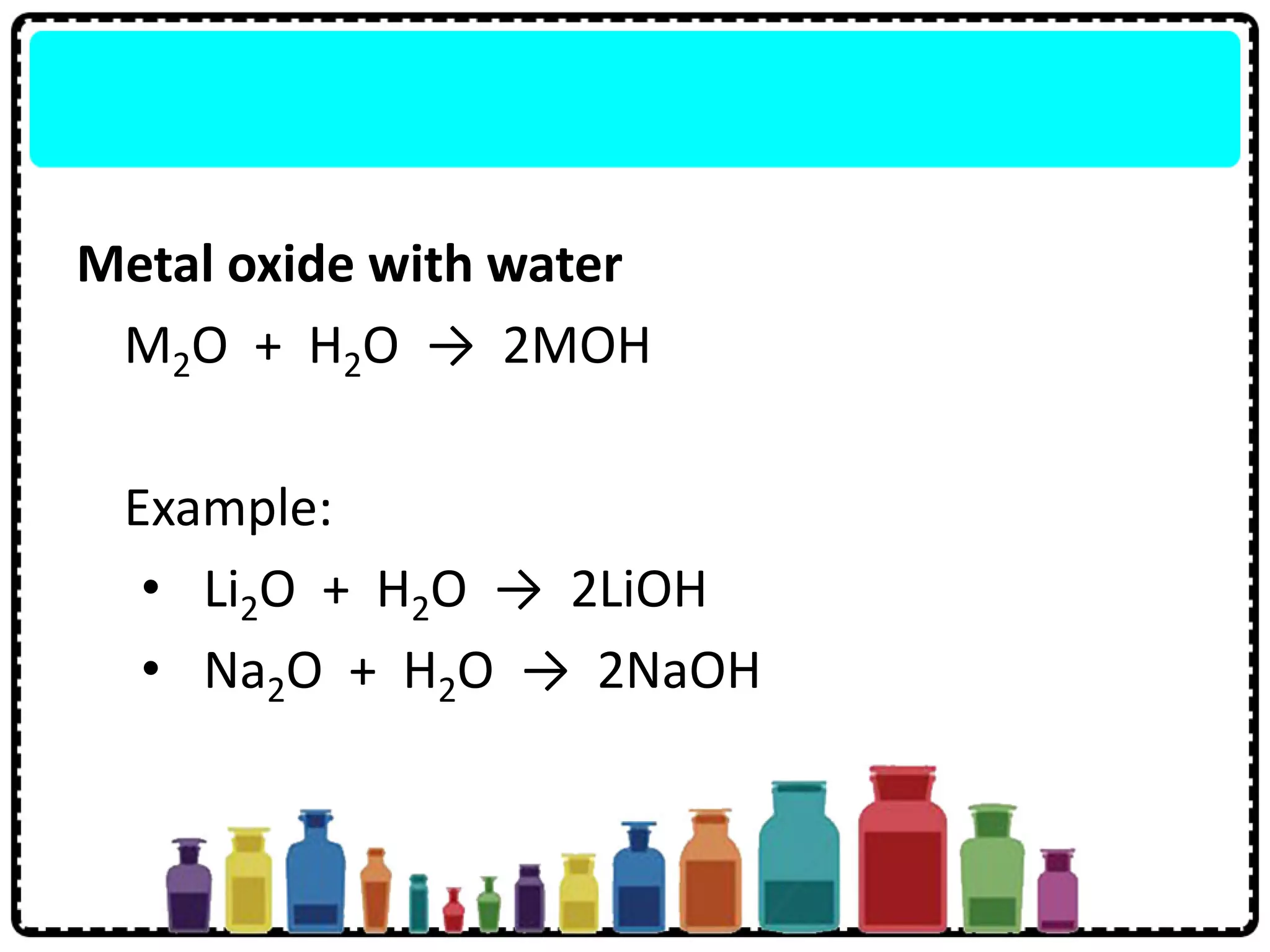
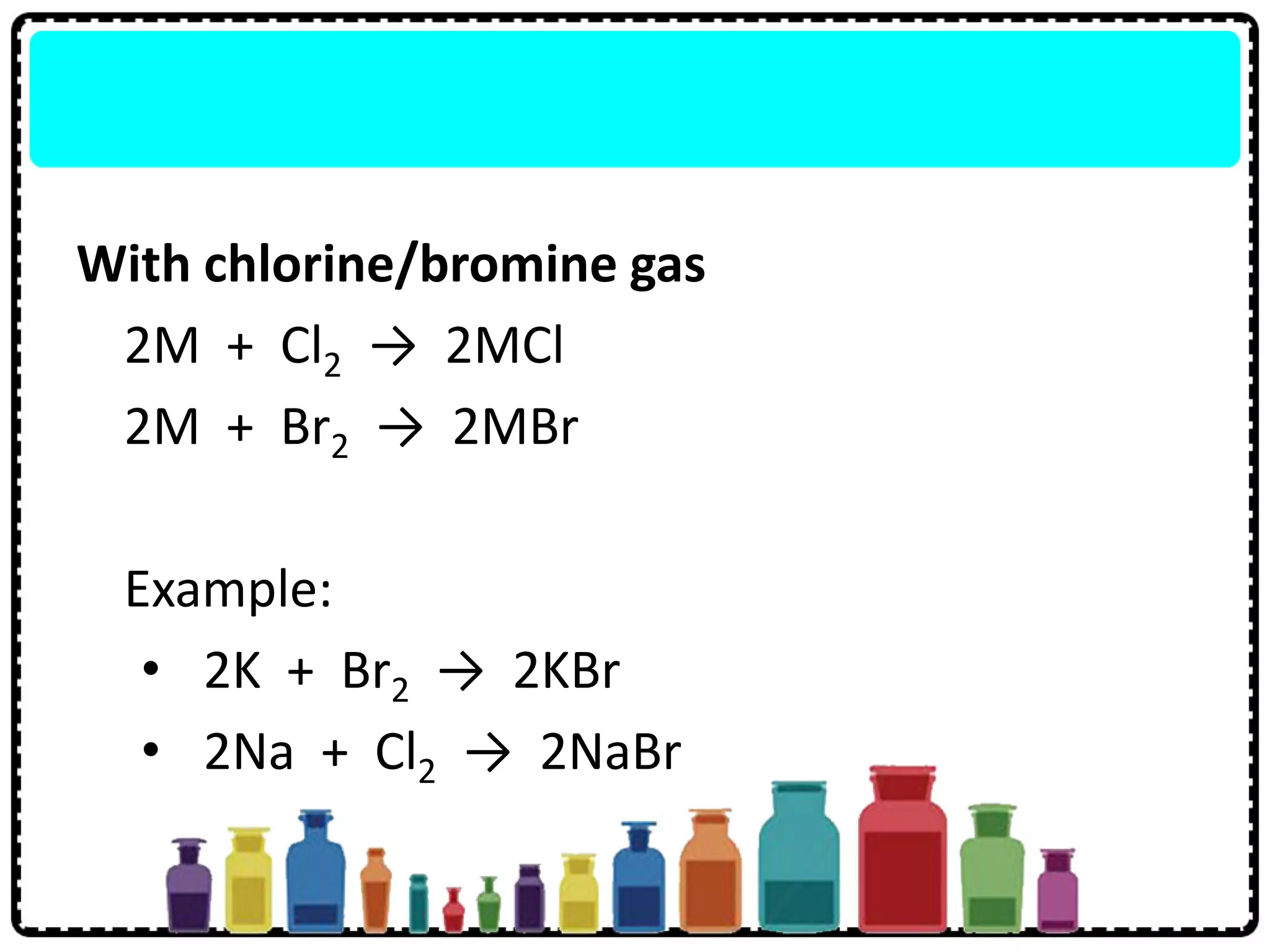
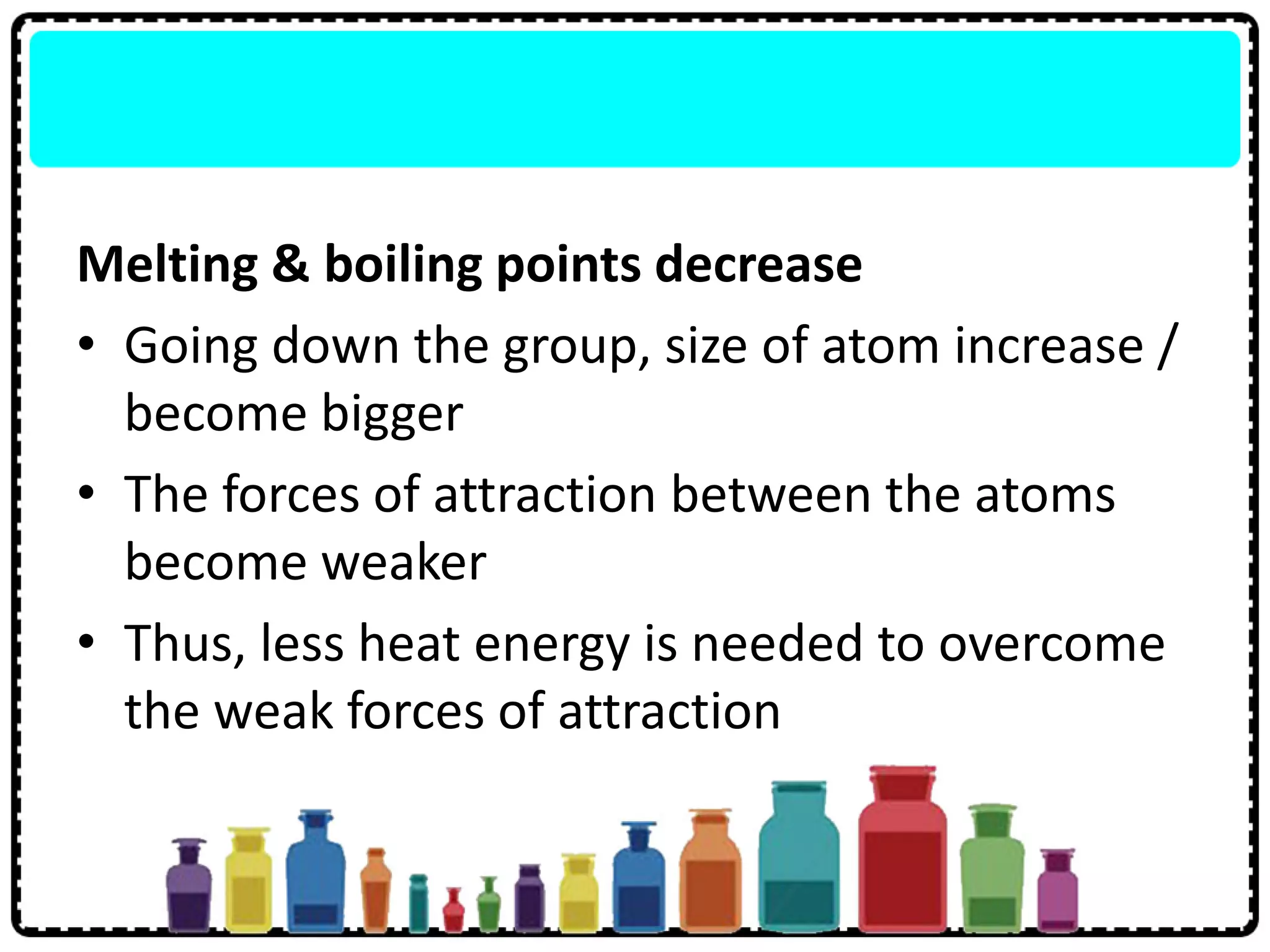
3) Group 1 elements are alkali metals that react vigorously with water and oxygen and reactivity increases down the group as atomic size increases.
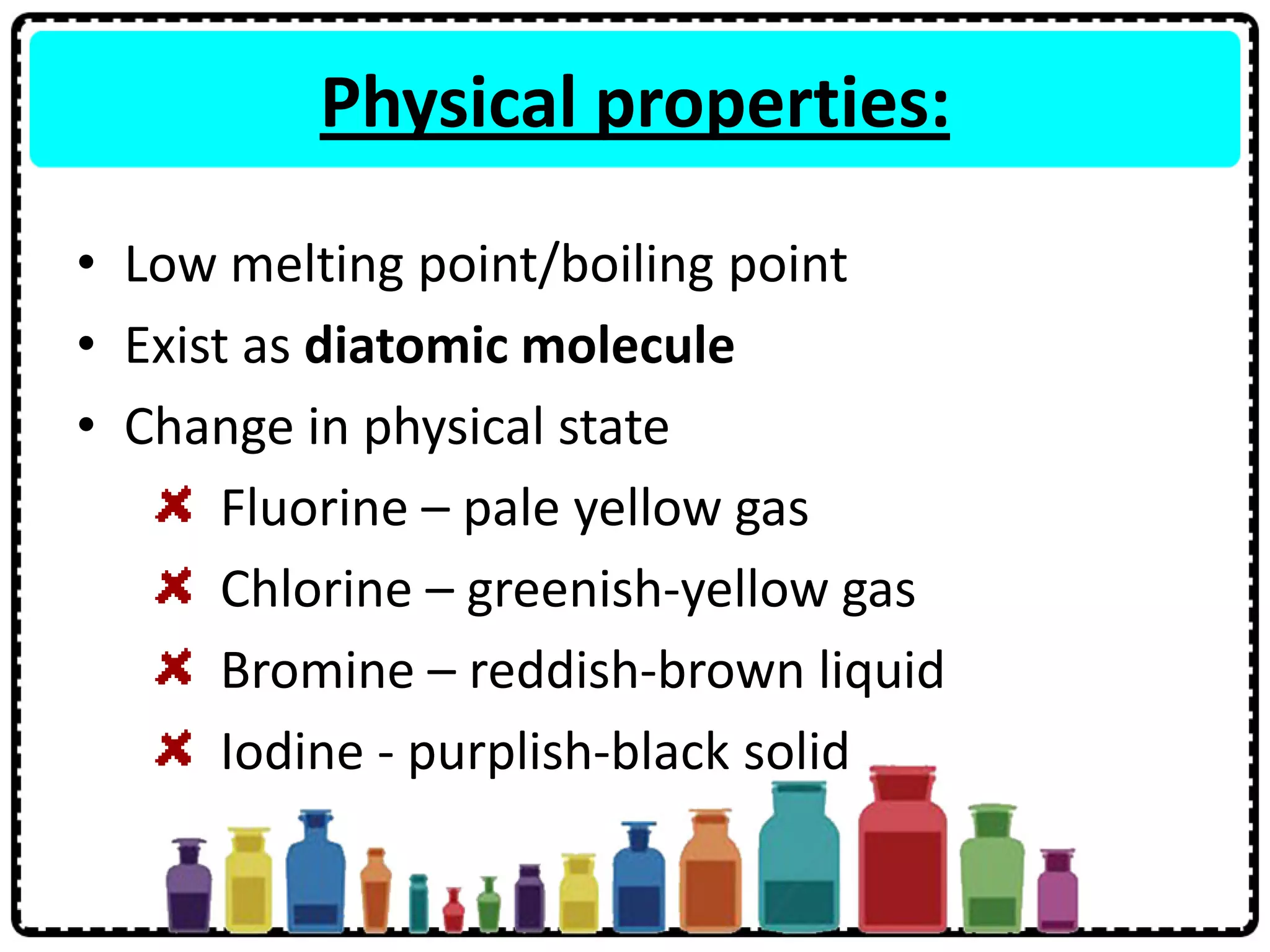
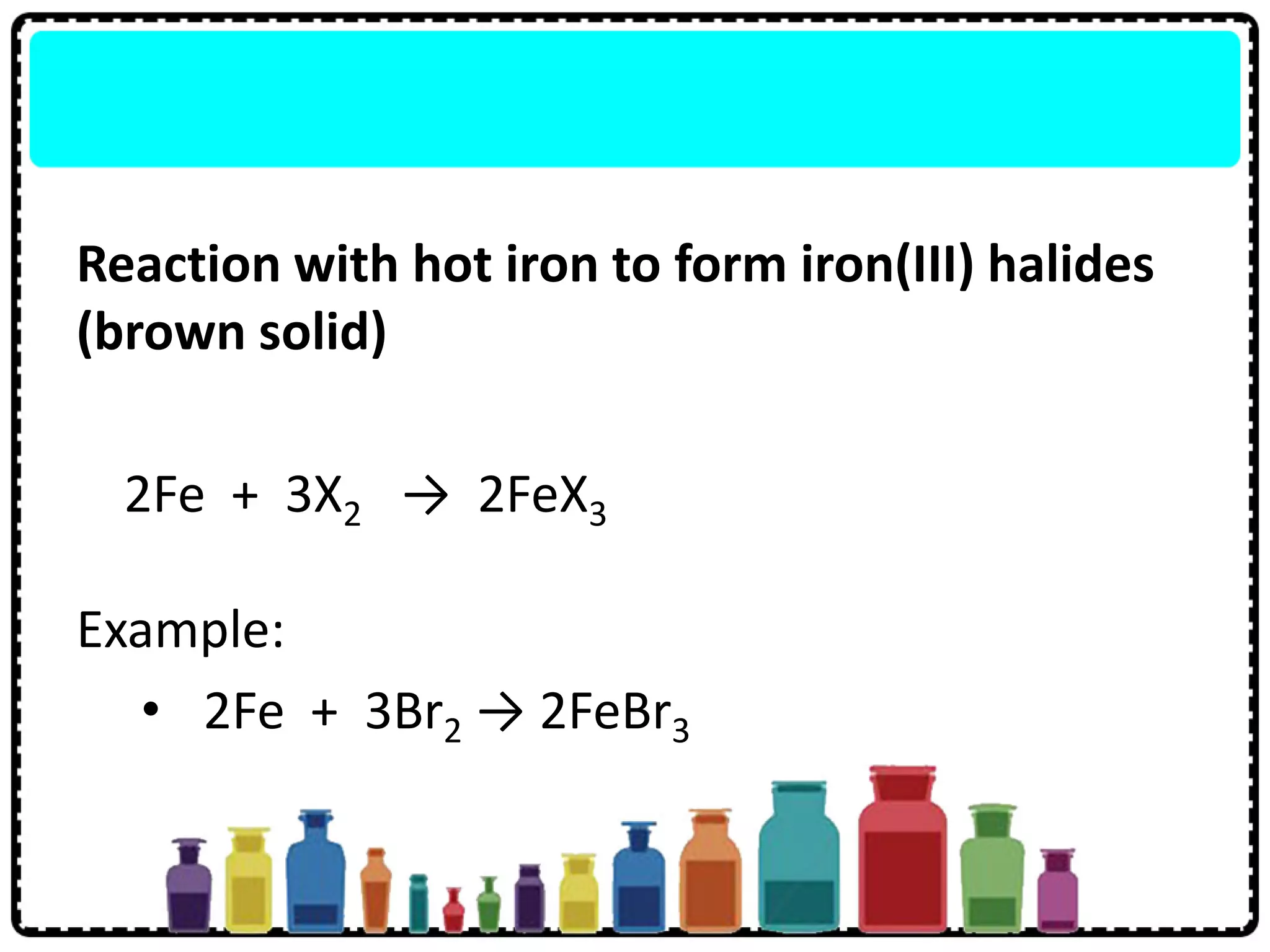
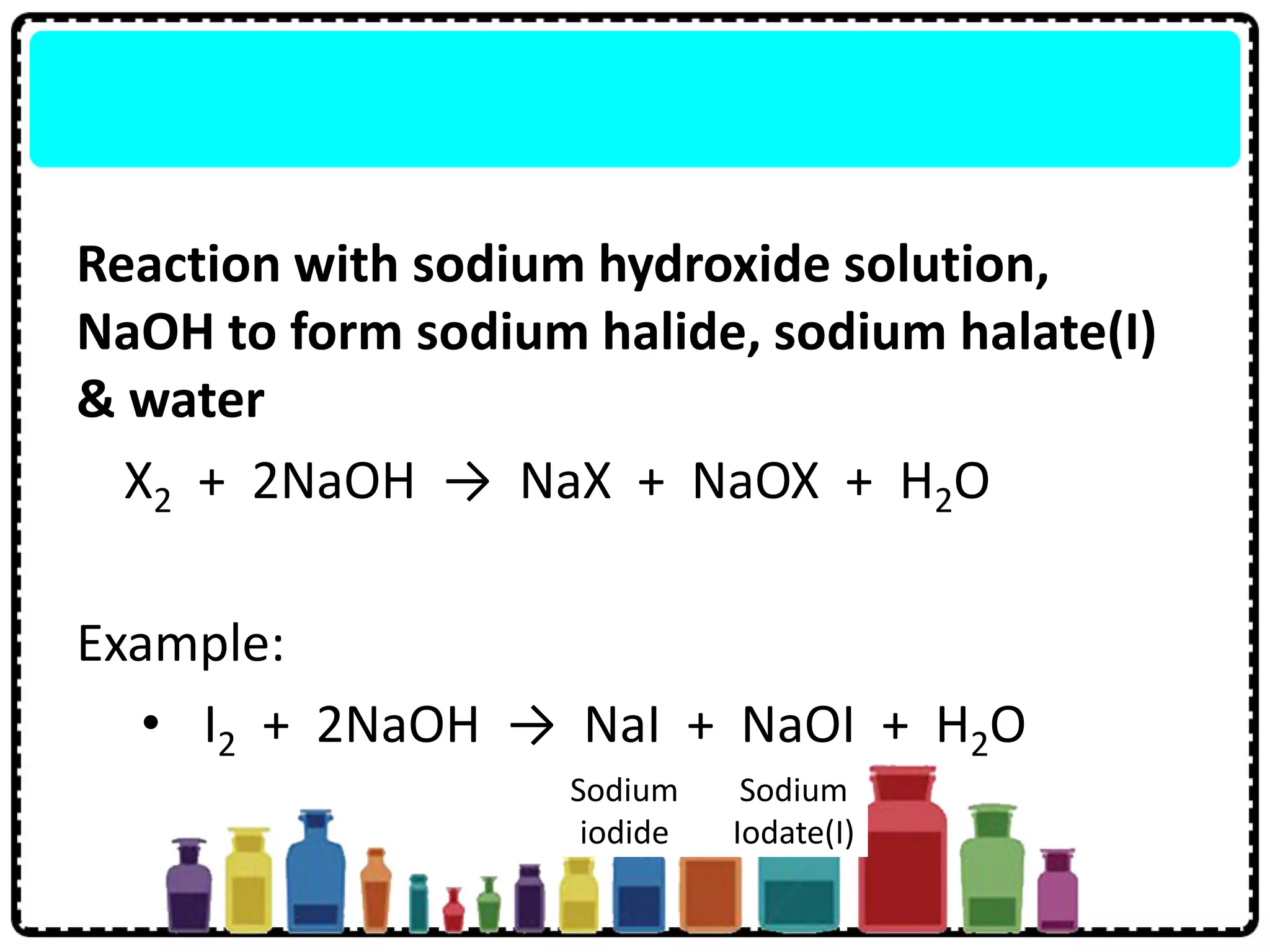
4) Group 17 elements are halogens that exist as diatomic molecules and reactivity decreases down the group as atomic size increases.
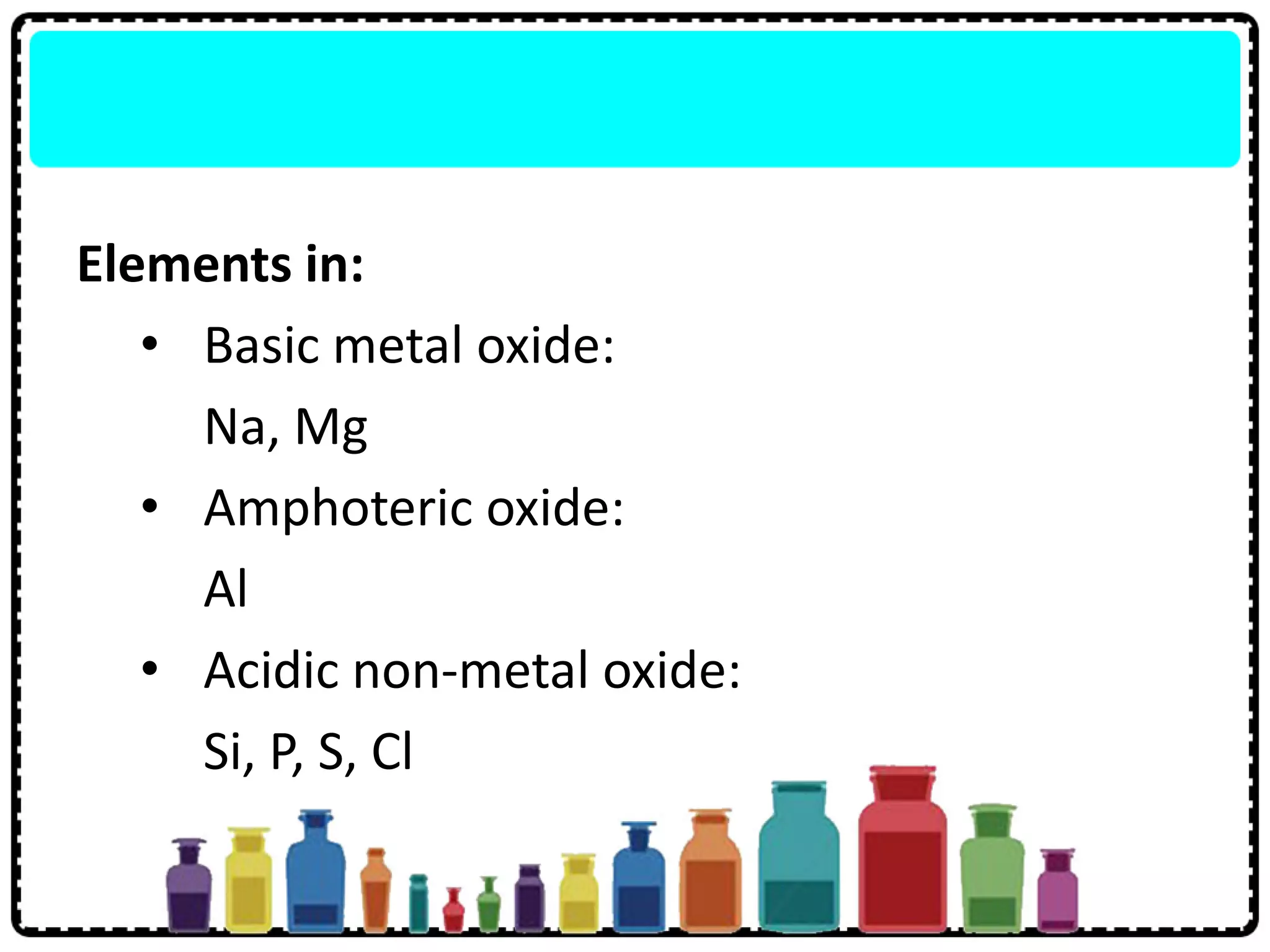
5) Period 3 elements include metals, nonmetals and semi-metals that show trends