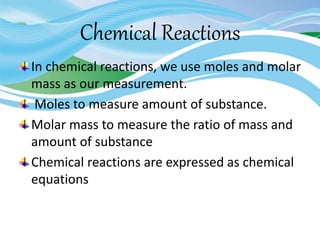
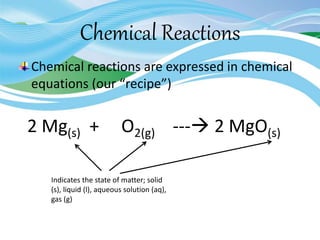
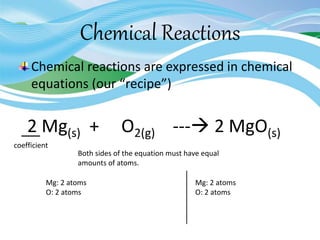
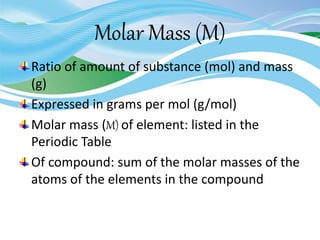
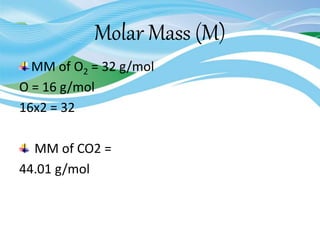
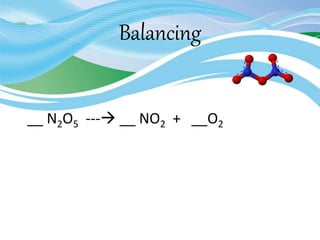
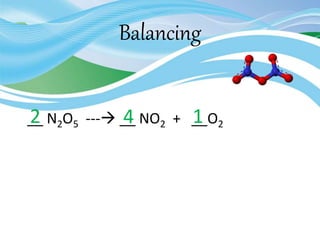
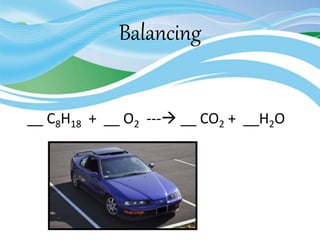
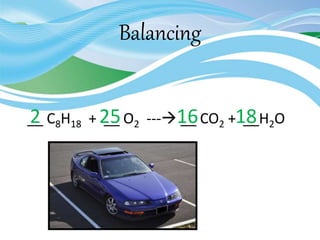
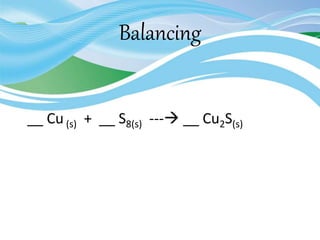
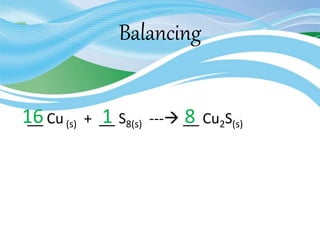
Stoichiometry is the study of quantitative relationships in chemical formulas and reactions. It uses moles and molar mass to quantify reactants and products. Chemical reactions are balanced and expressed as equations. Stoichiometric problems involve calculating amounts in moles and grams for limiting reactants, conversions between moles and mass, and determining theoretical yields.

![Stoichiometry
Stoicheion [Gr. “element” or “part”]
Metron [Gr. “measure”]
Study of quantitative aspects of chemical
formulas and reactions](https://image.slidesharecdn.com/chemistrystoichiometry-200828050319/85/Chemistry-stoichiometry-3-320.jpg)








![The Mole (mol)
SI unit for amount of substance
Defined as the amount of a substance that
contains the same number of entities as there are
atoms in exactly 12 grams of carbon-12. That is
6.022 x1023 [called Avogrado’s number]
∴ 1 mol of carbon-12 contains 6.022 x1023
carbon-12 atoms
1 mol of H2O contains 6.022 x1023 H2O molecules](https://image.slidesharecdn.com/chemistrystoichiometry-200828050319/85/Chemistry-stoichiometry-15-320.jpg)









![Sources
• Dragon Cave Holiday Cooking 2012. [Photos]
• Silberberg, Martin S. “Stoichiometry of Formulas and
Equations”. Principles of General Chemistry. McGraw-Hill,
2010.](https://image.slidesharecdn.com/chemistrystoichiometry-200828050319/85/Chemistry-stoichiometry-37-320.jpg)