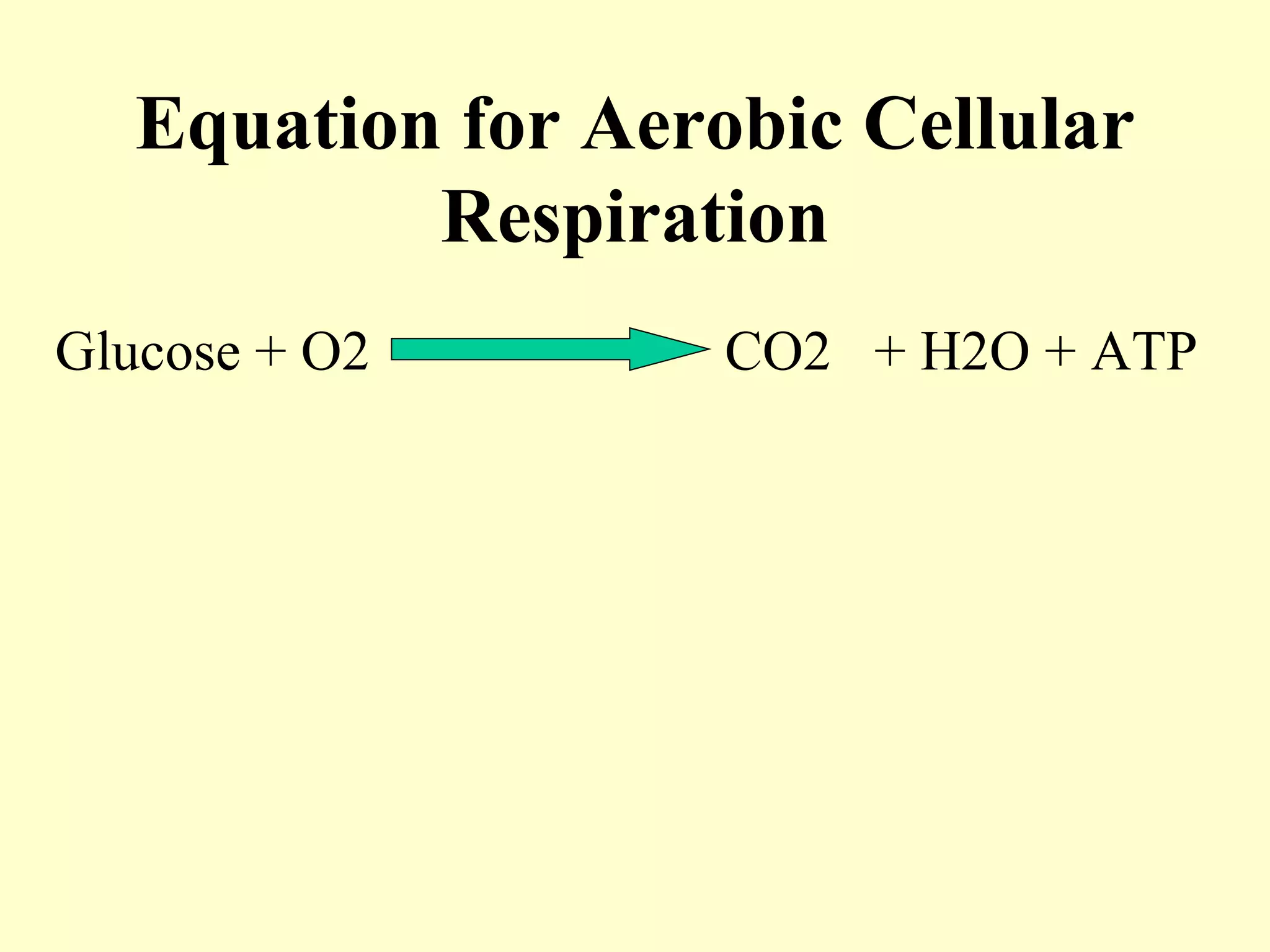
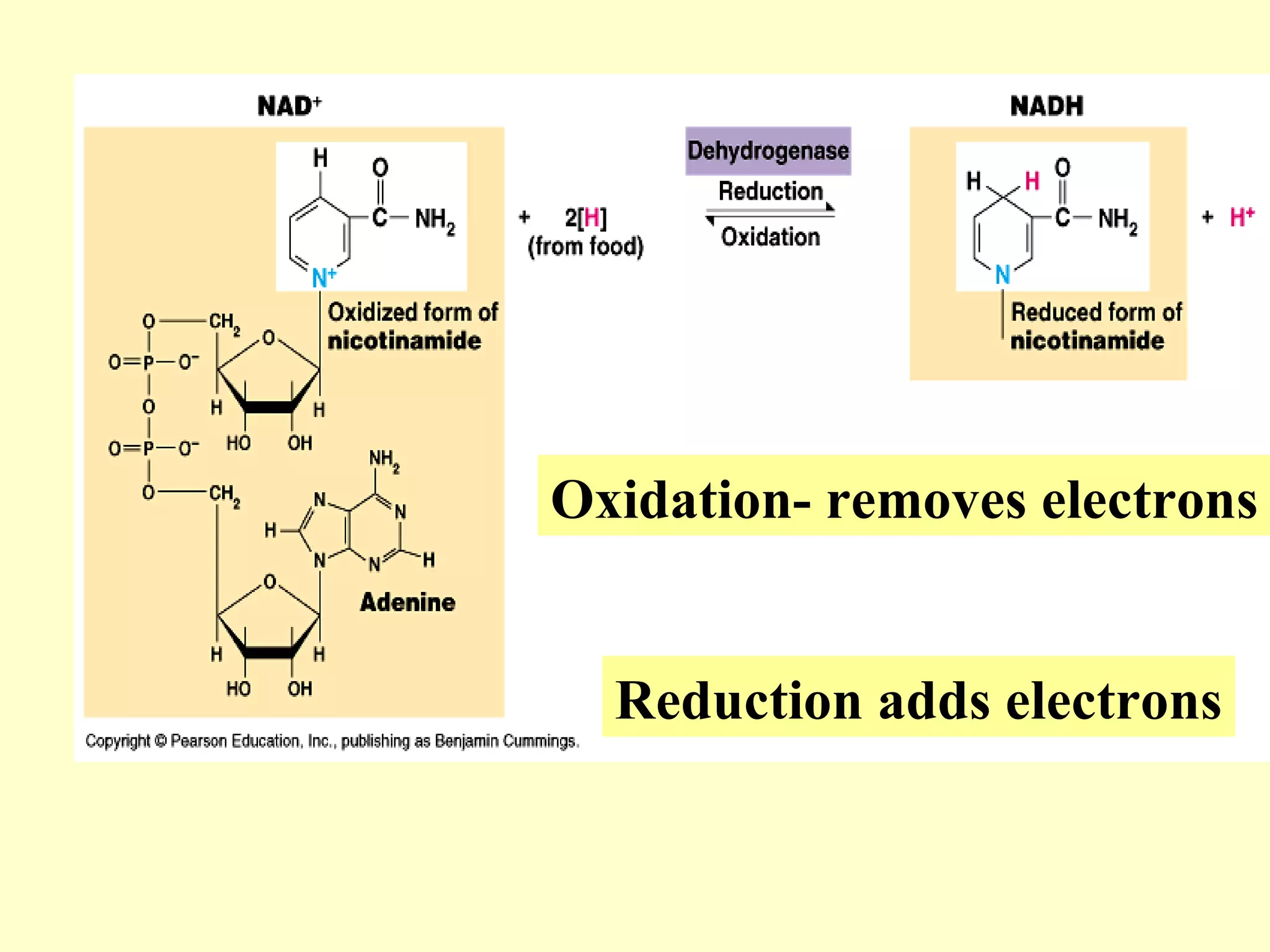
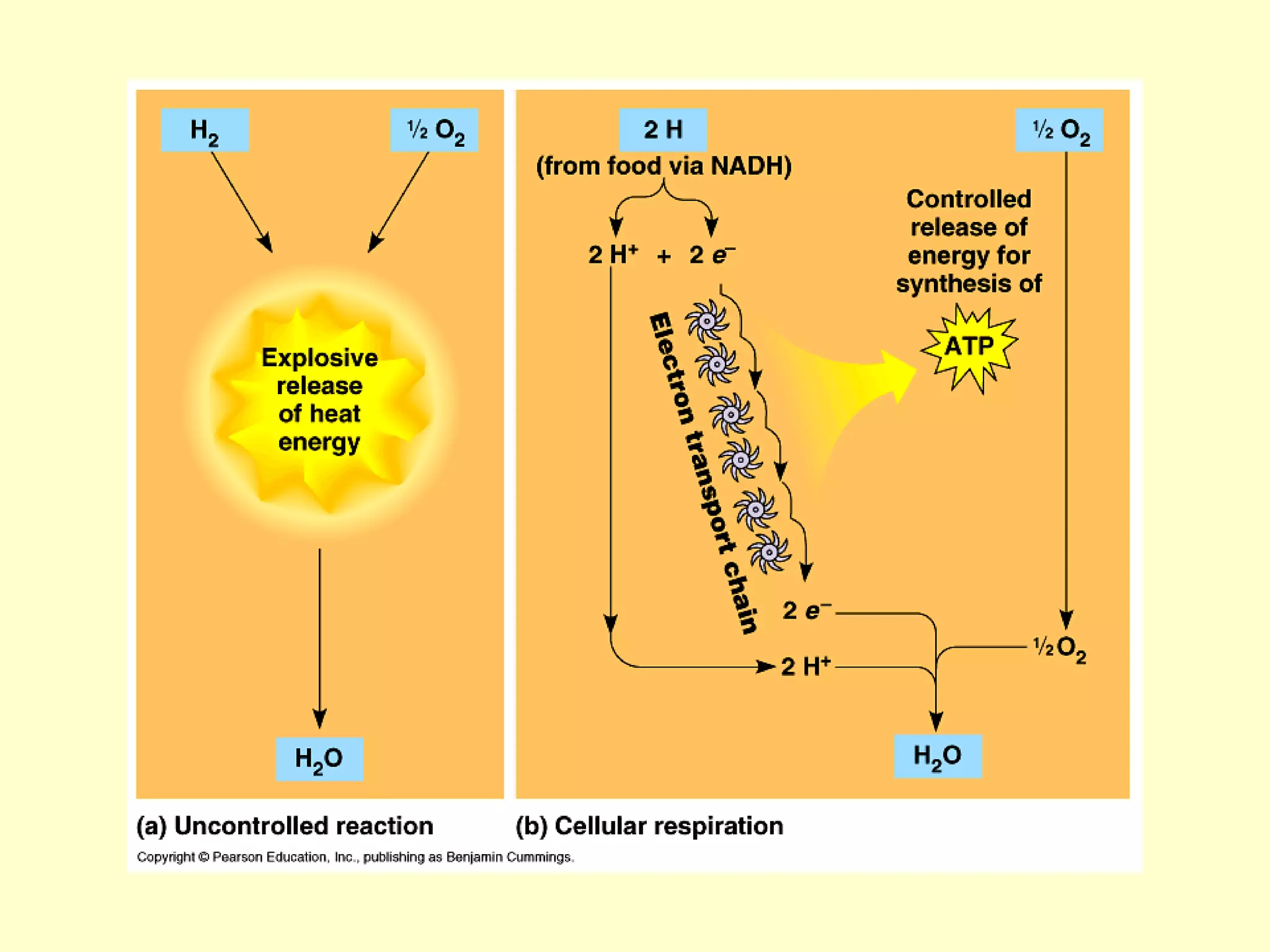
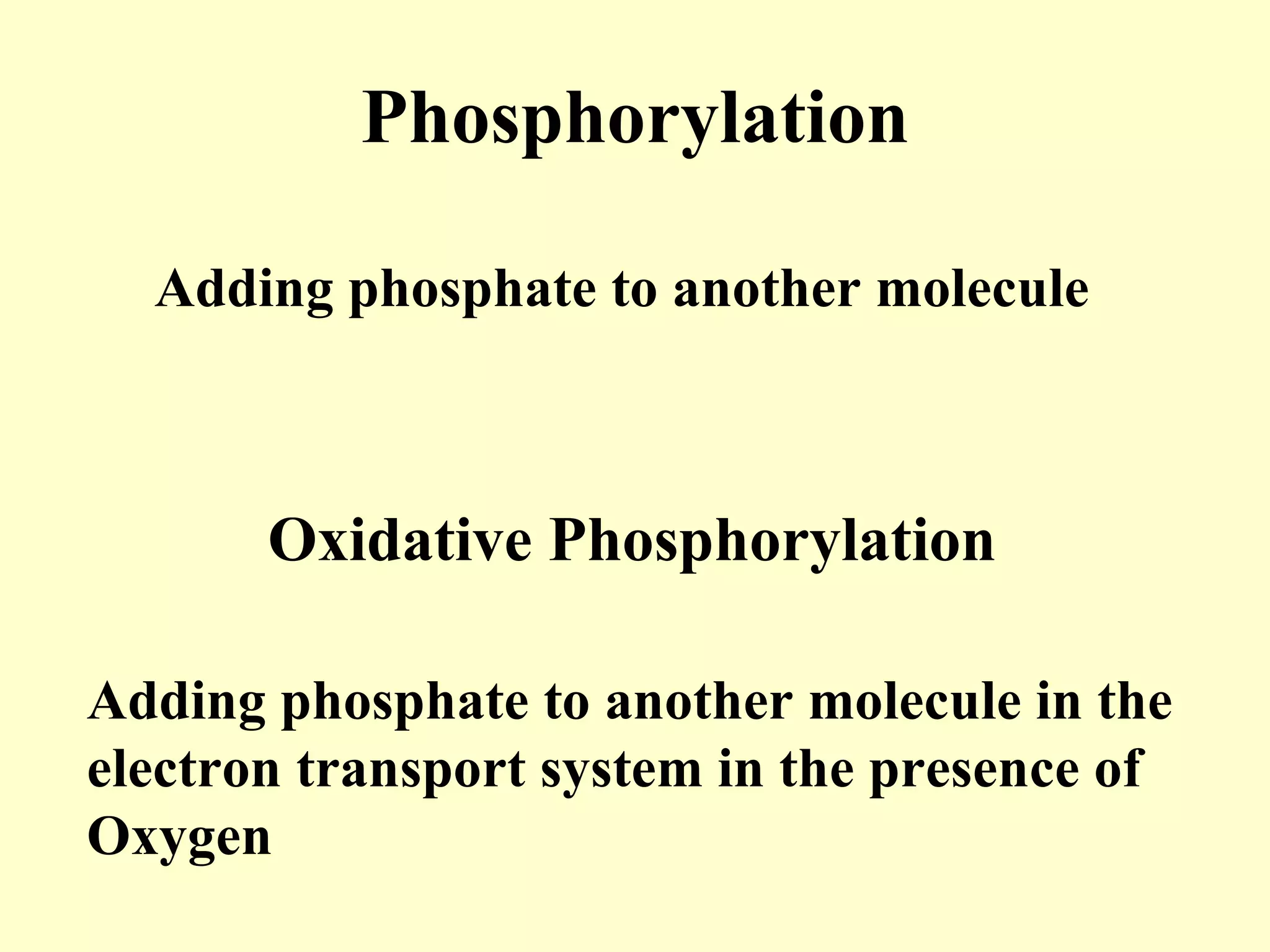
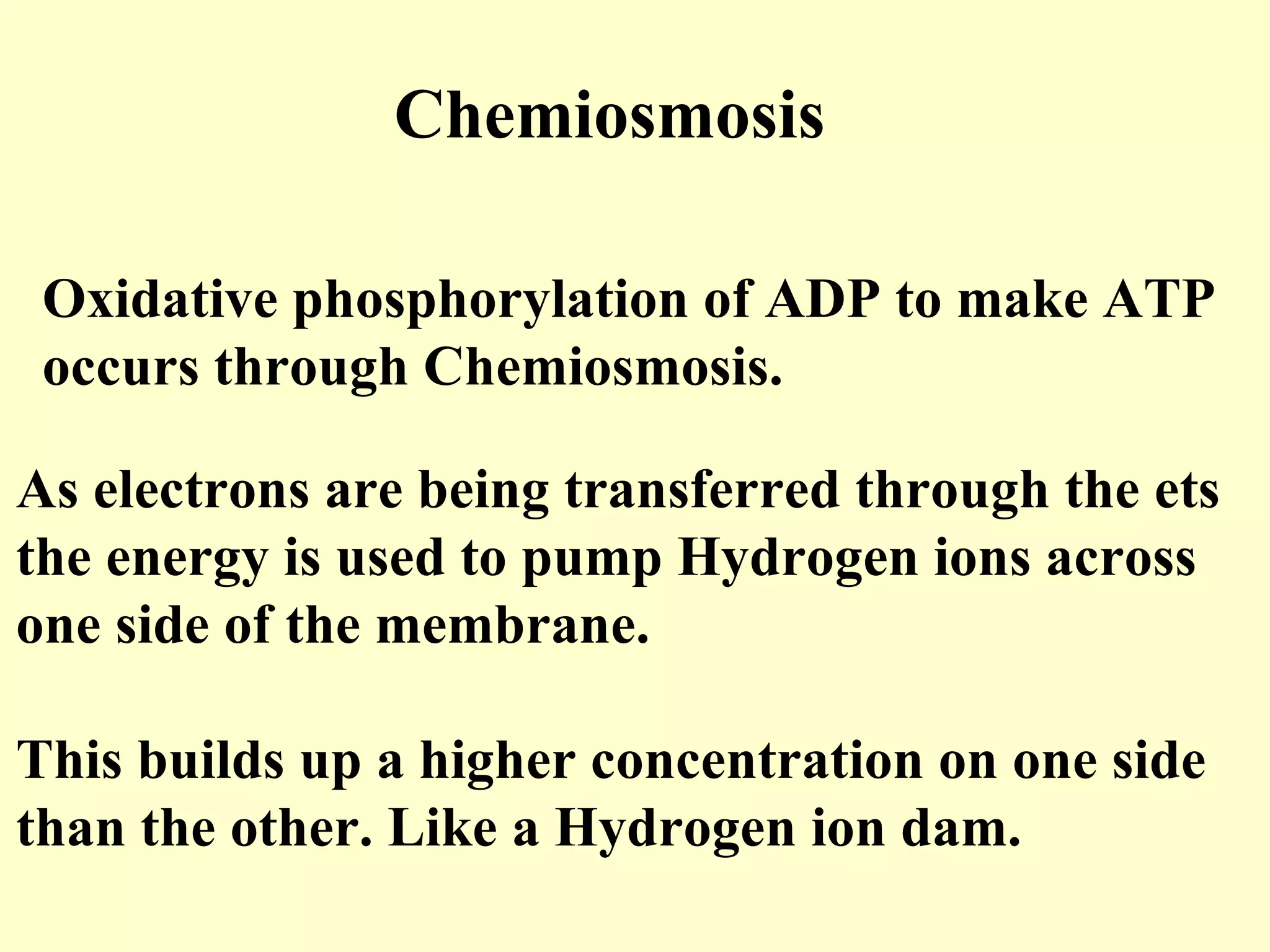
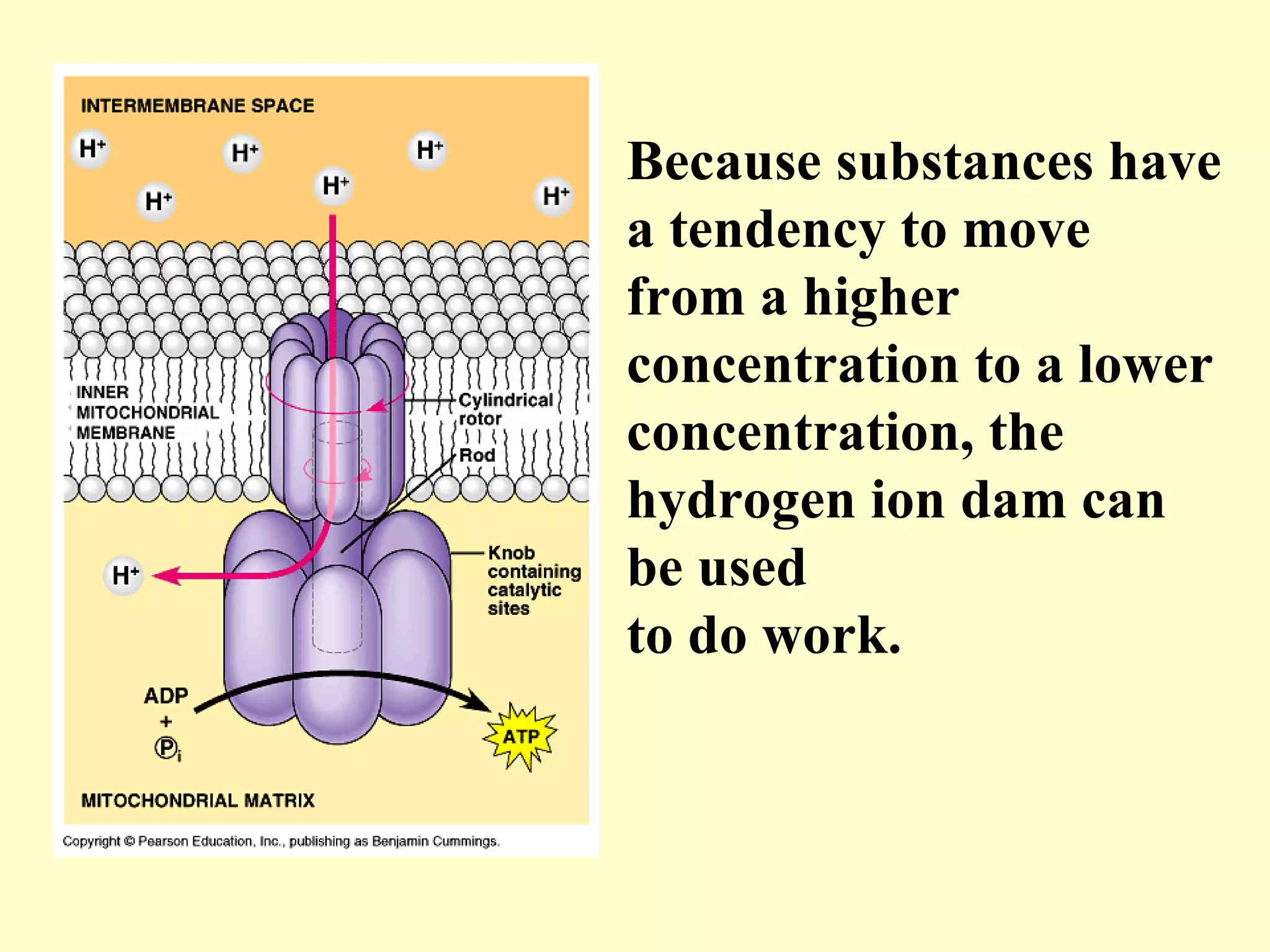
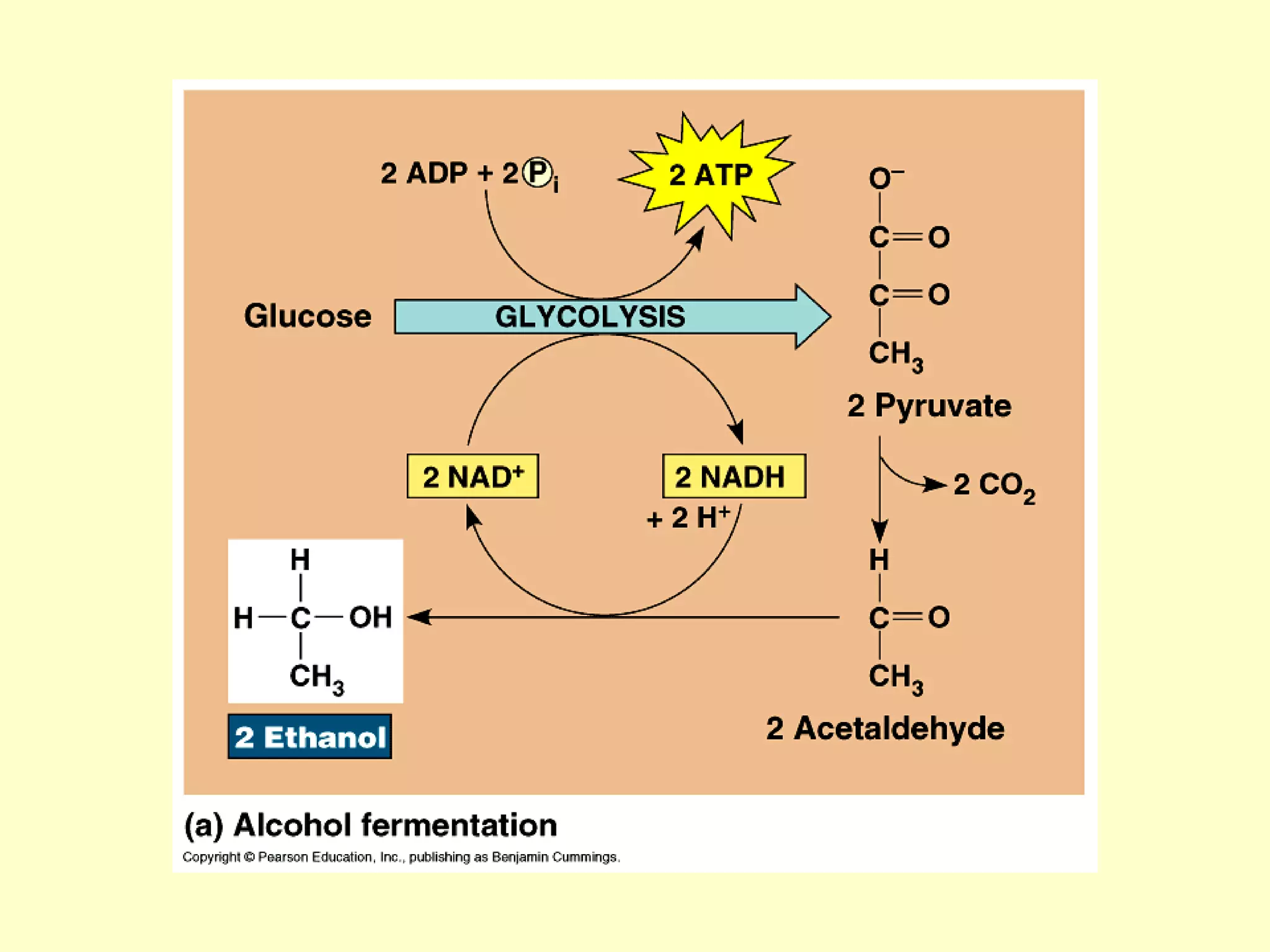
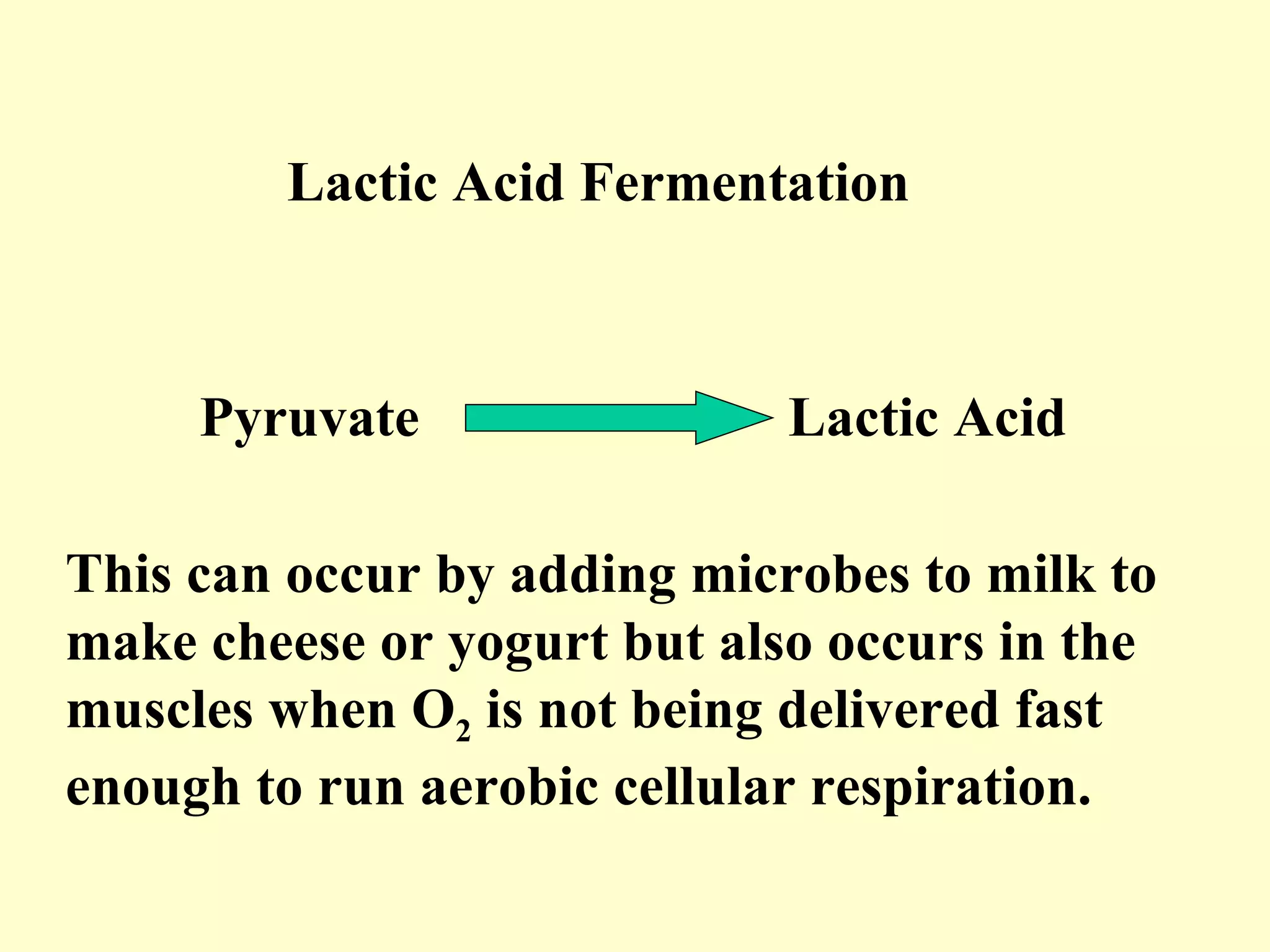
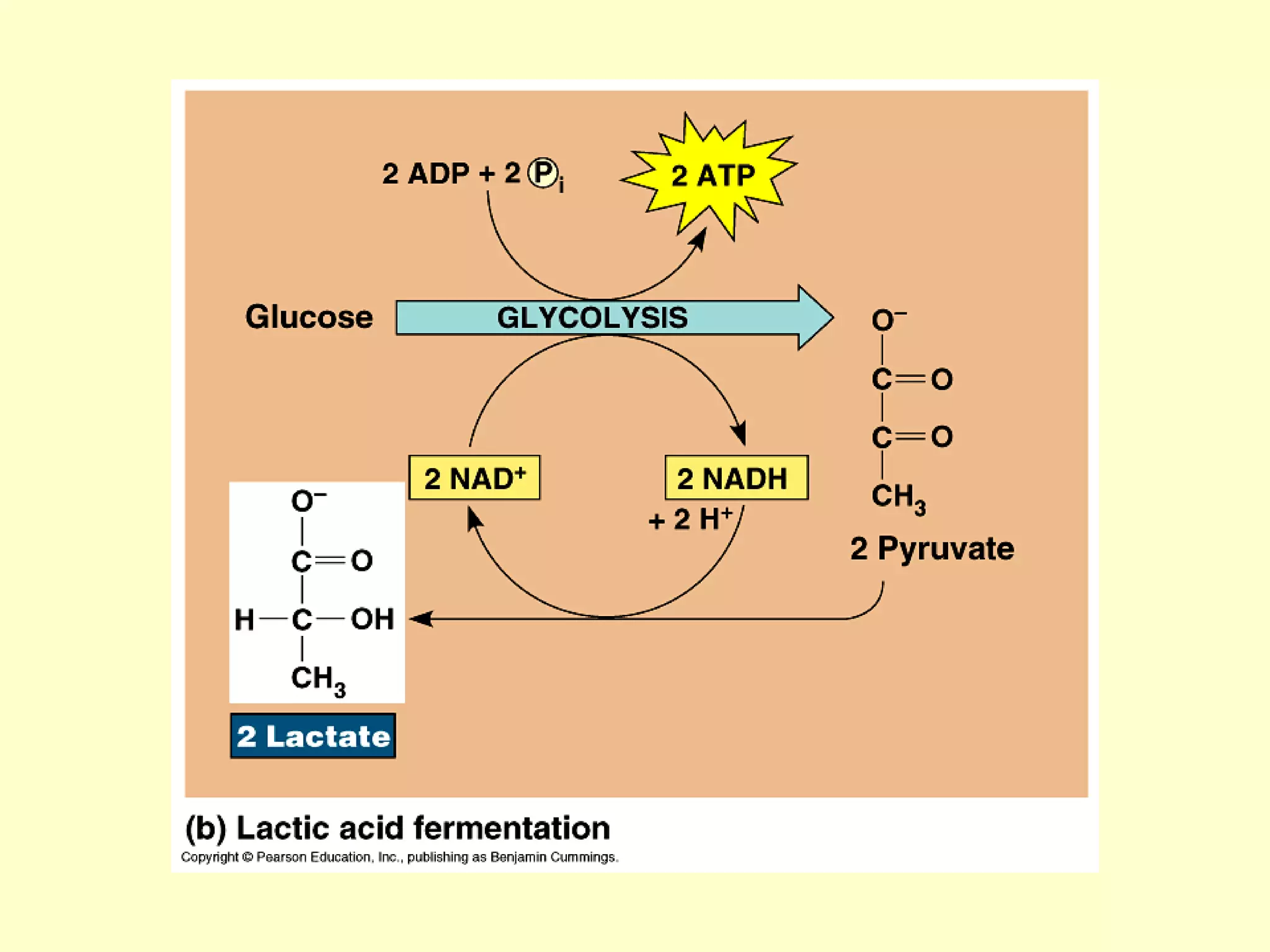
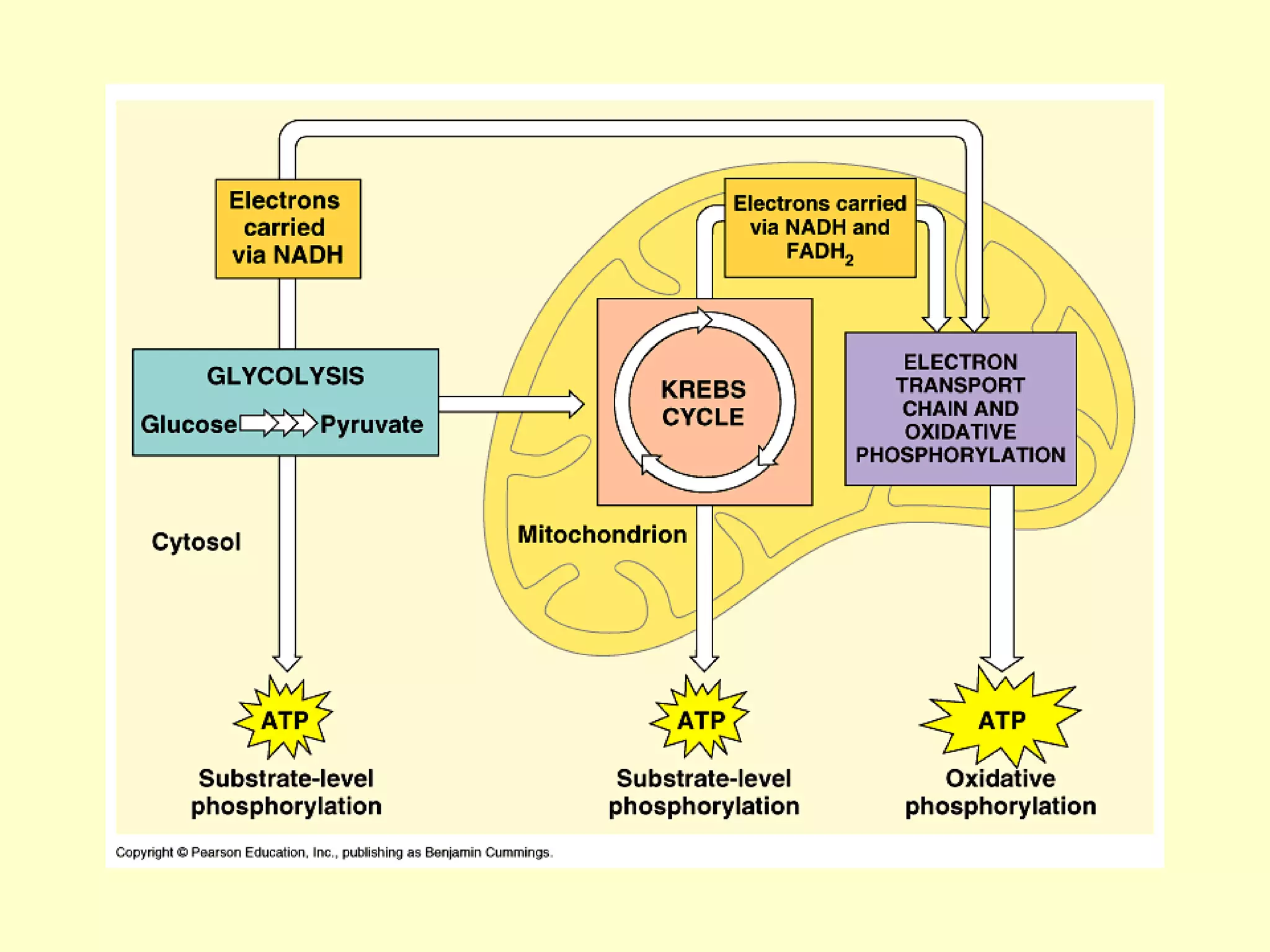
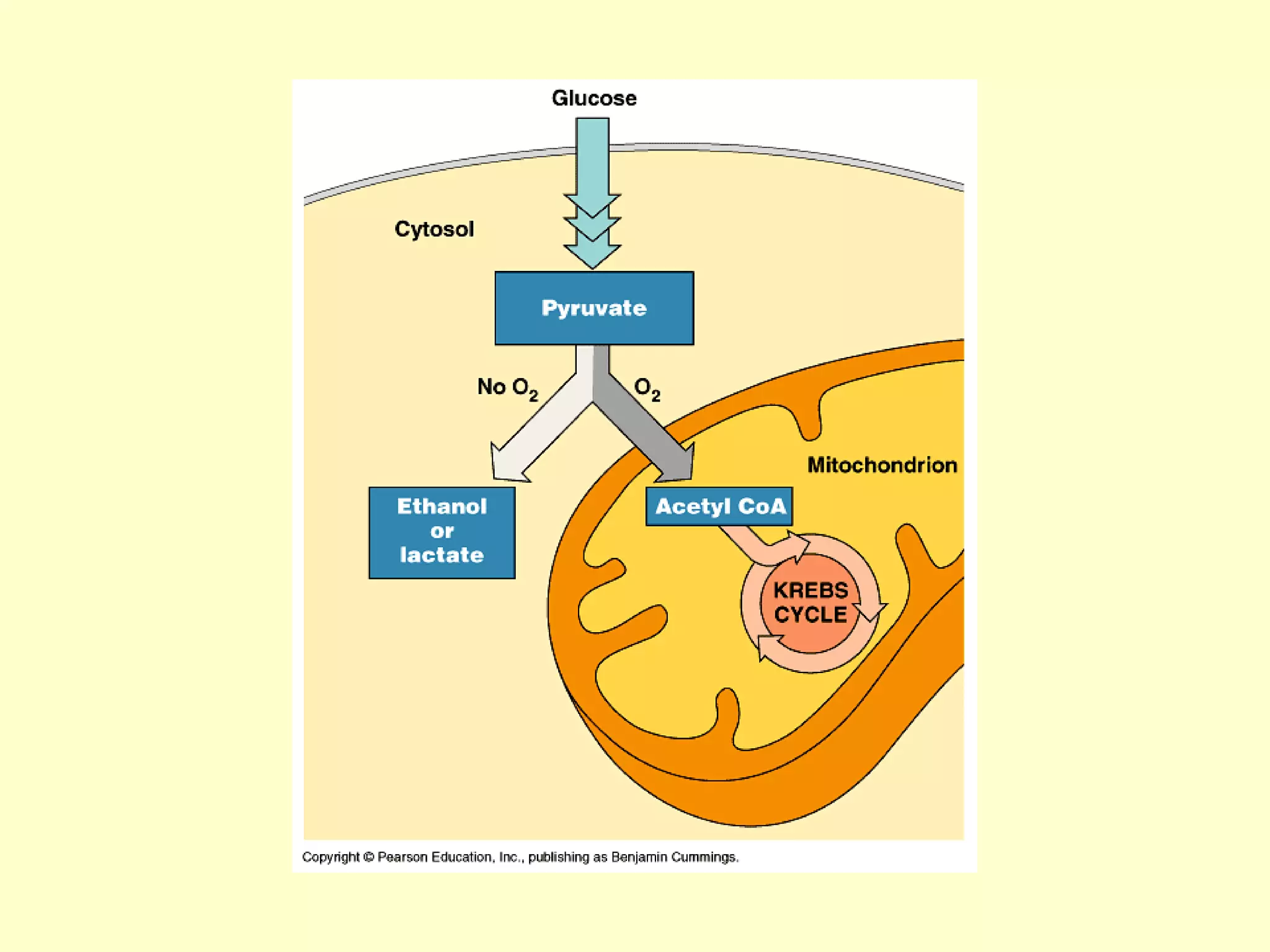
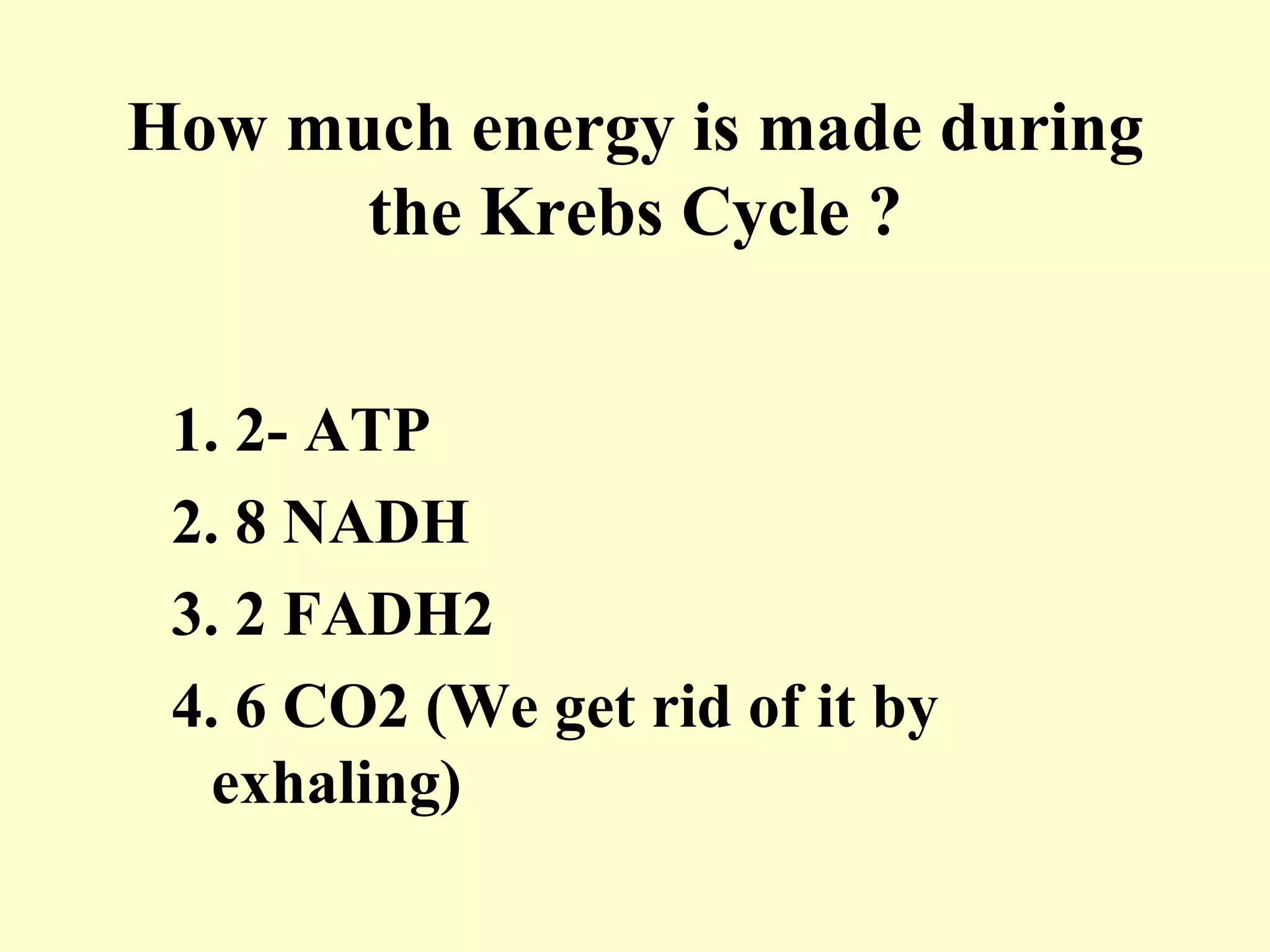1) Cellular respiration uses oxygen and food to produce energy in the form of ATP through a series of chemical reactions.
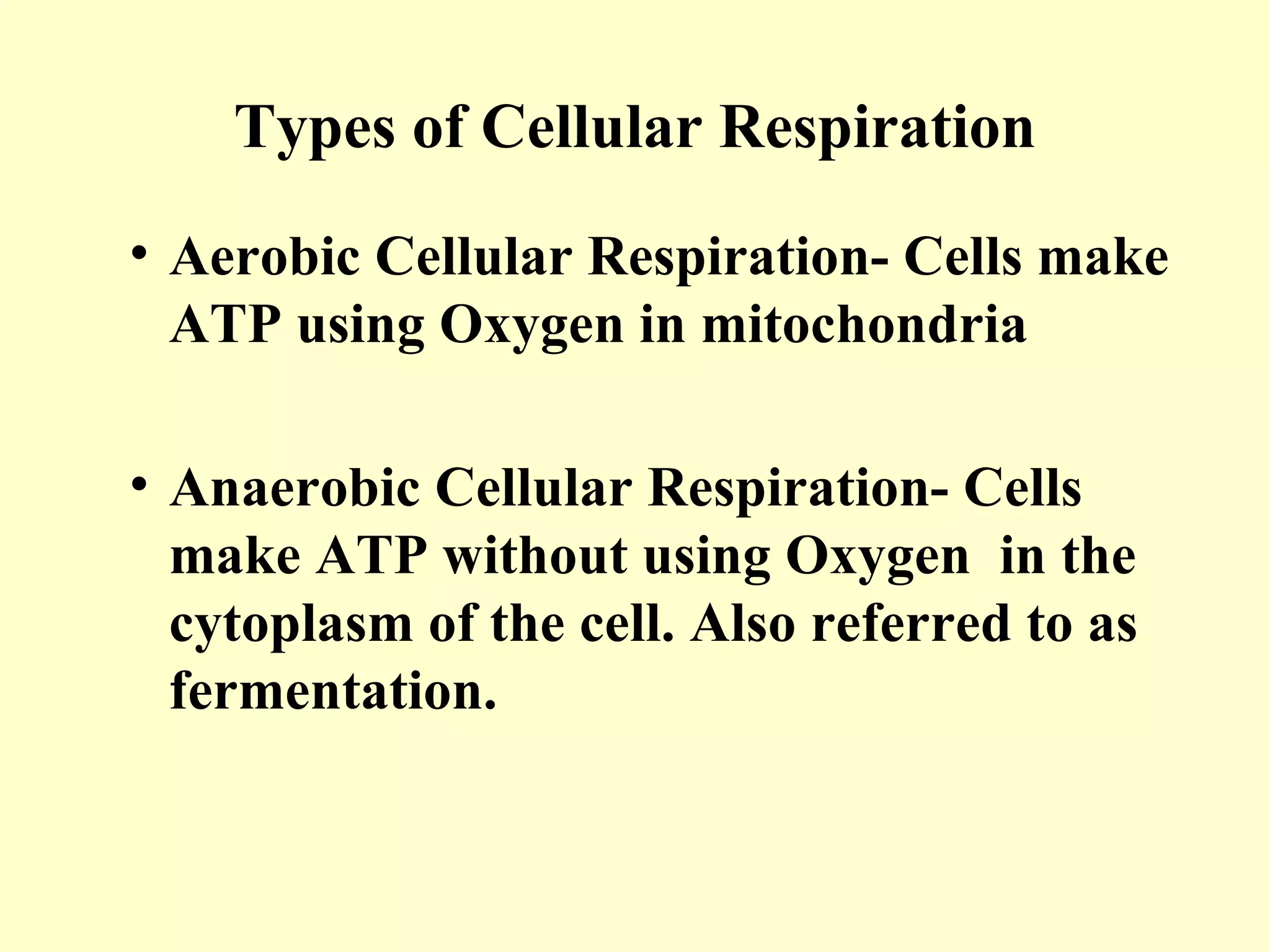
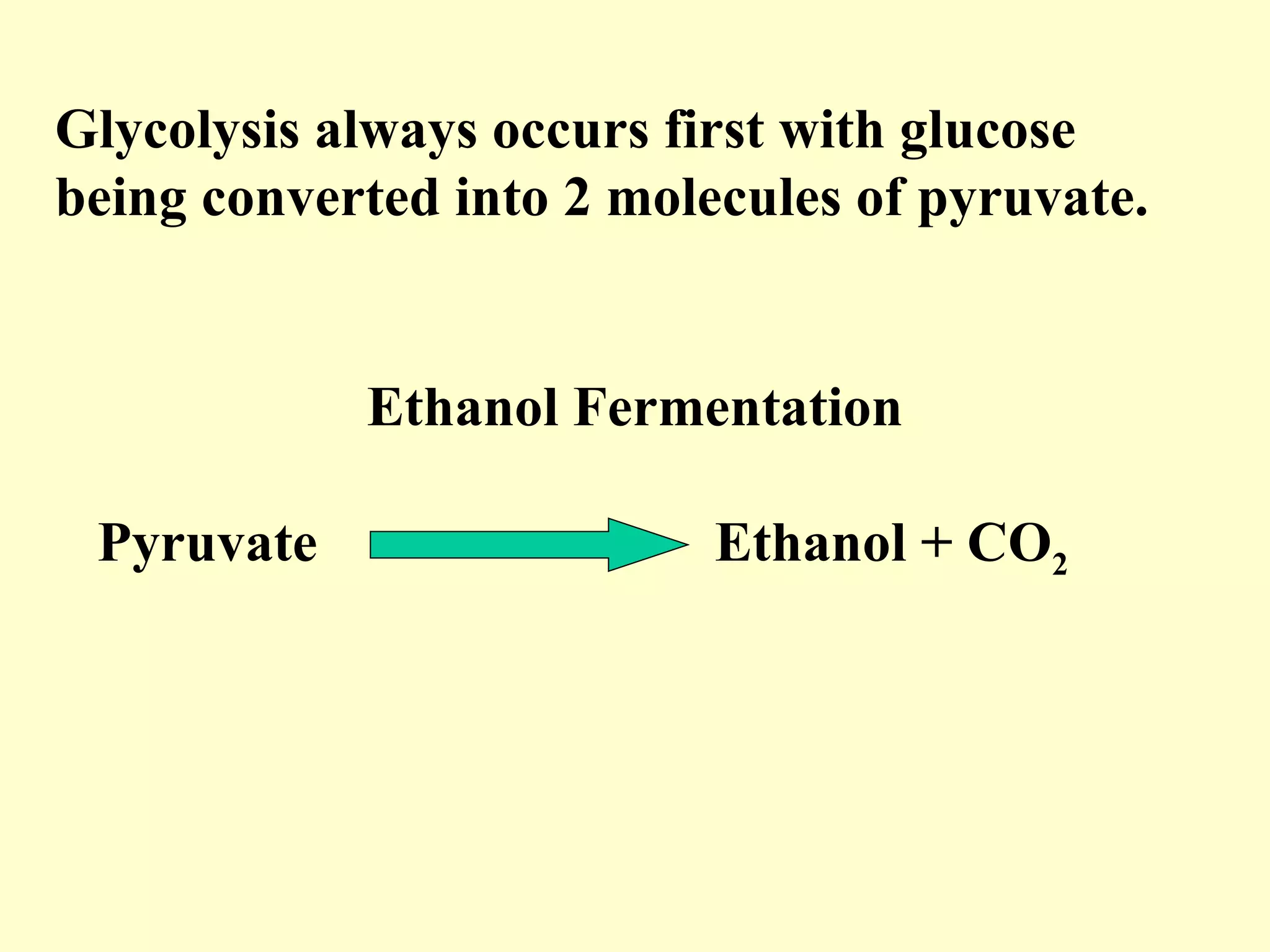
2) There are two types of cellular respiration: aerobic respiration which uses oxygen to produce 38 ATP and anaerobic respiration which produces only 2 ATP without oxygen.
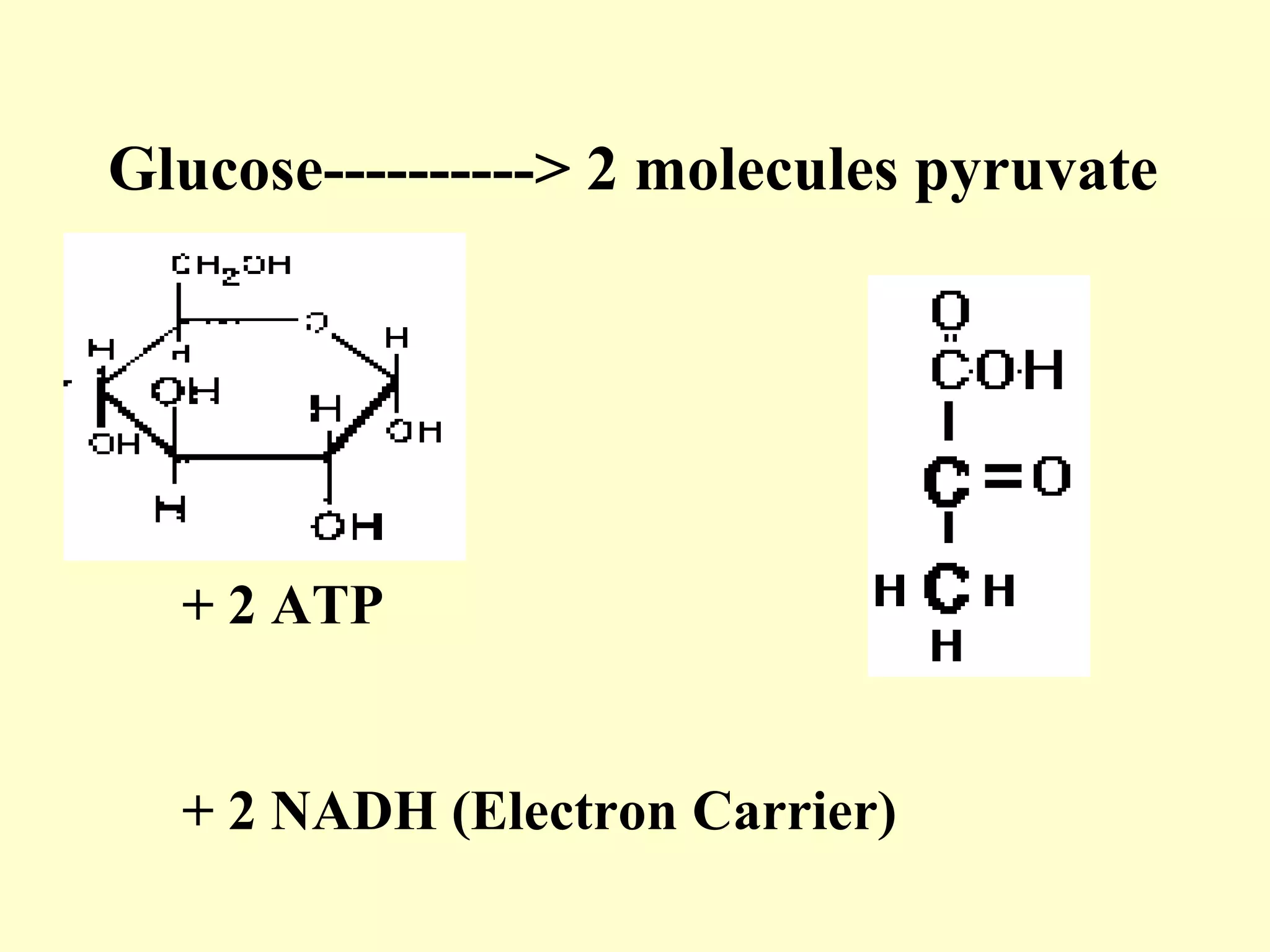
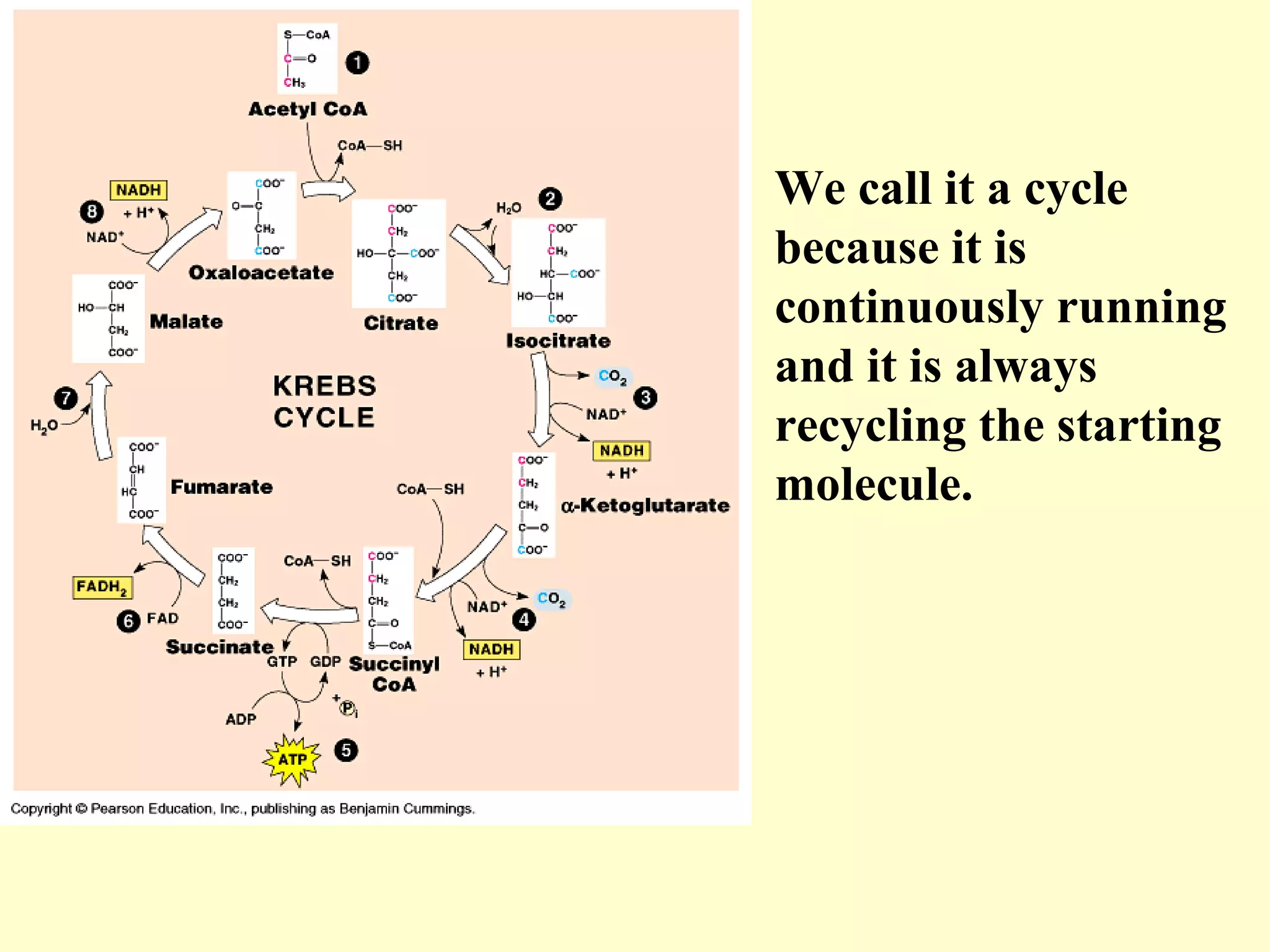
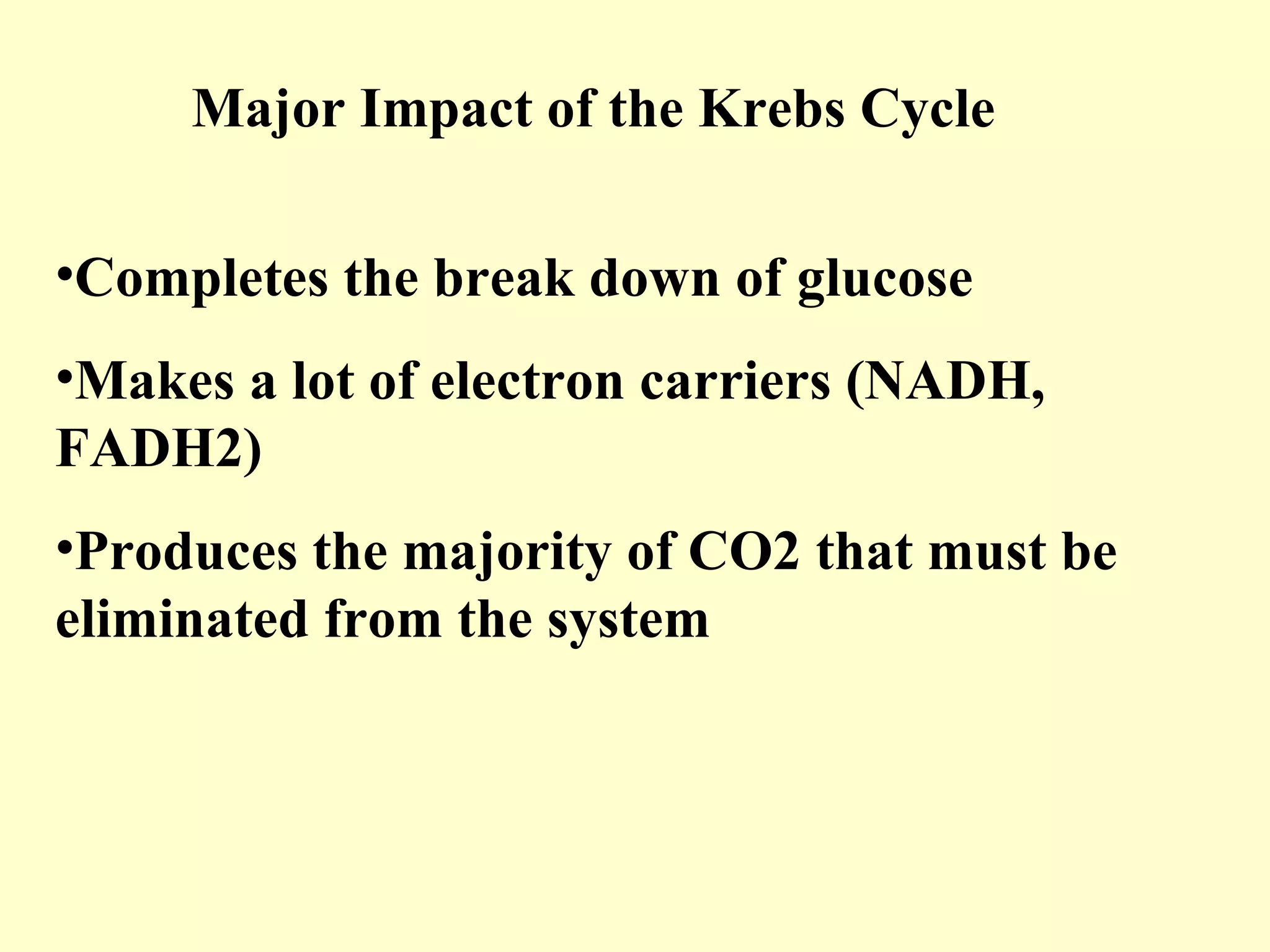
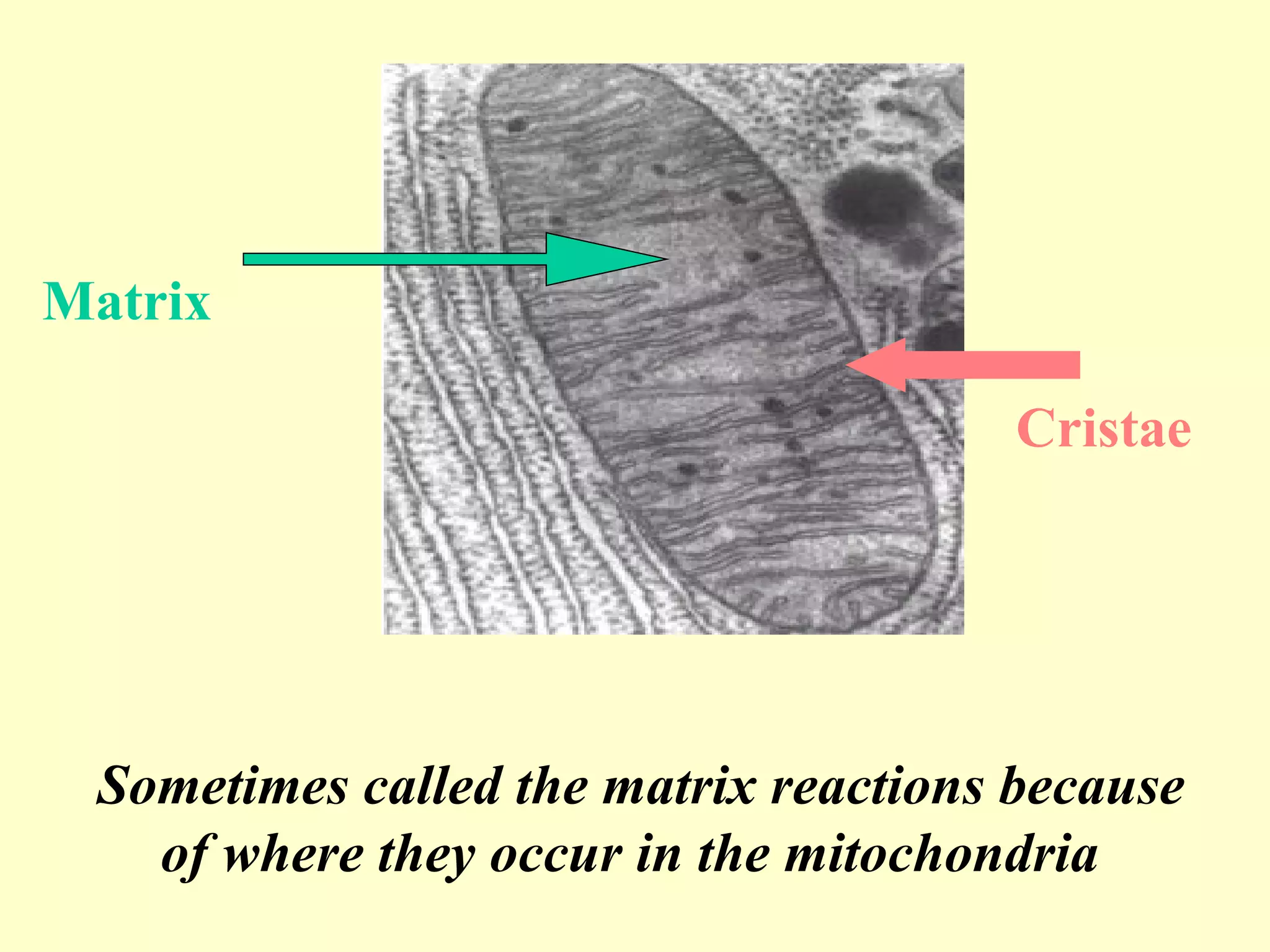
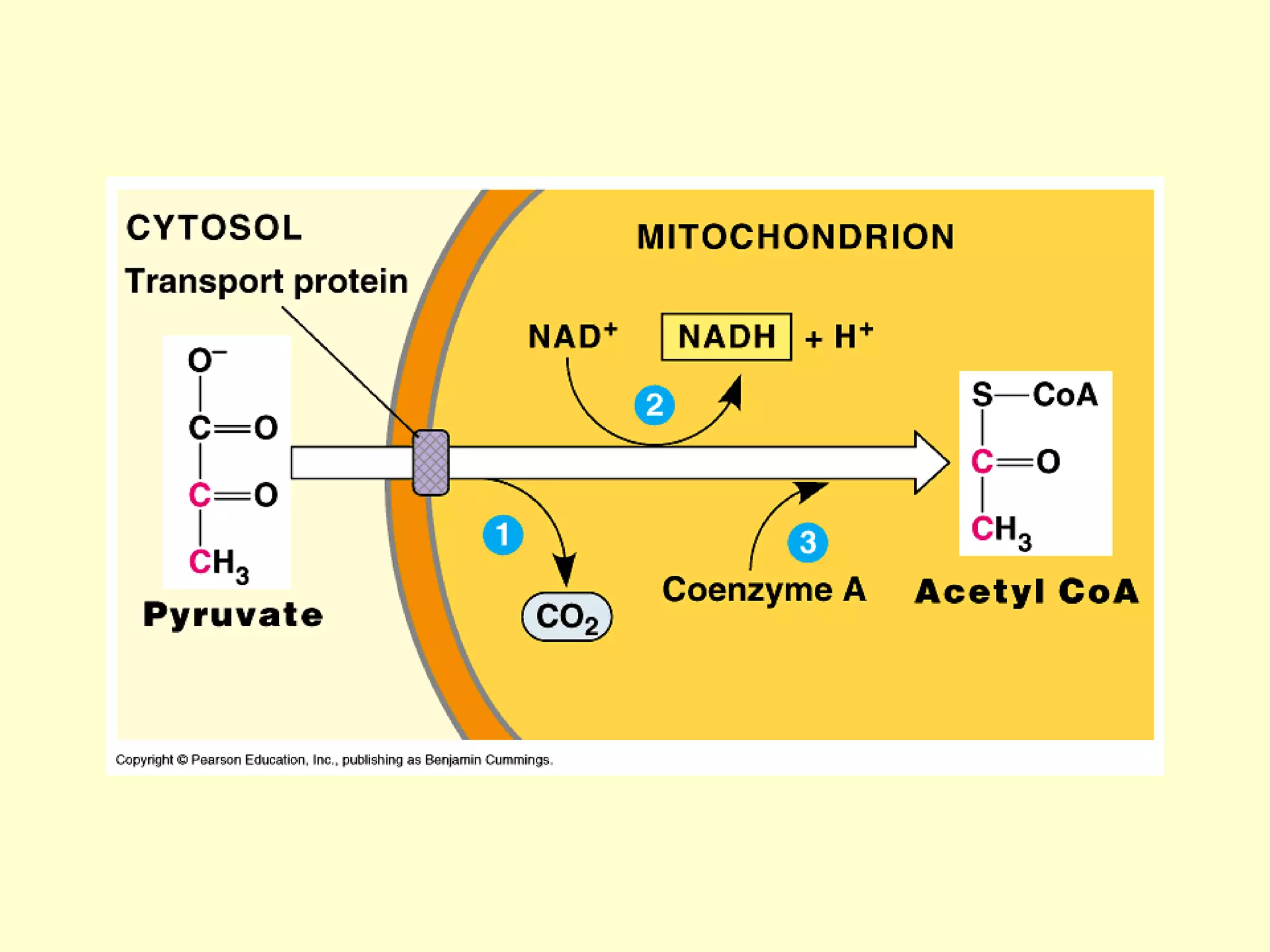
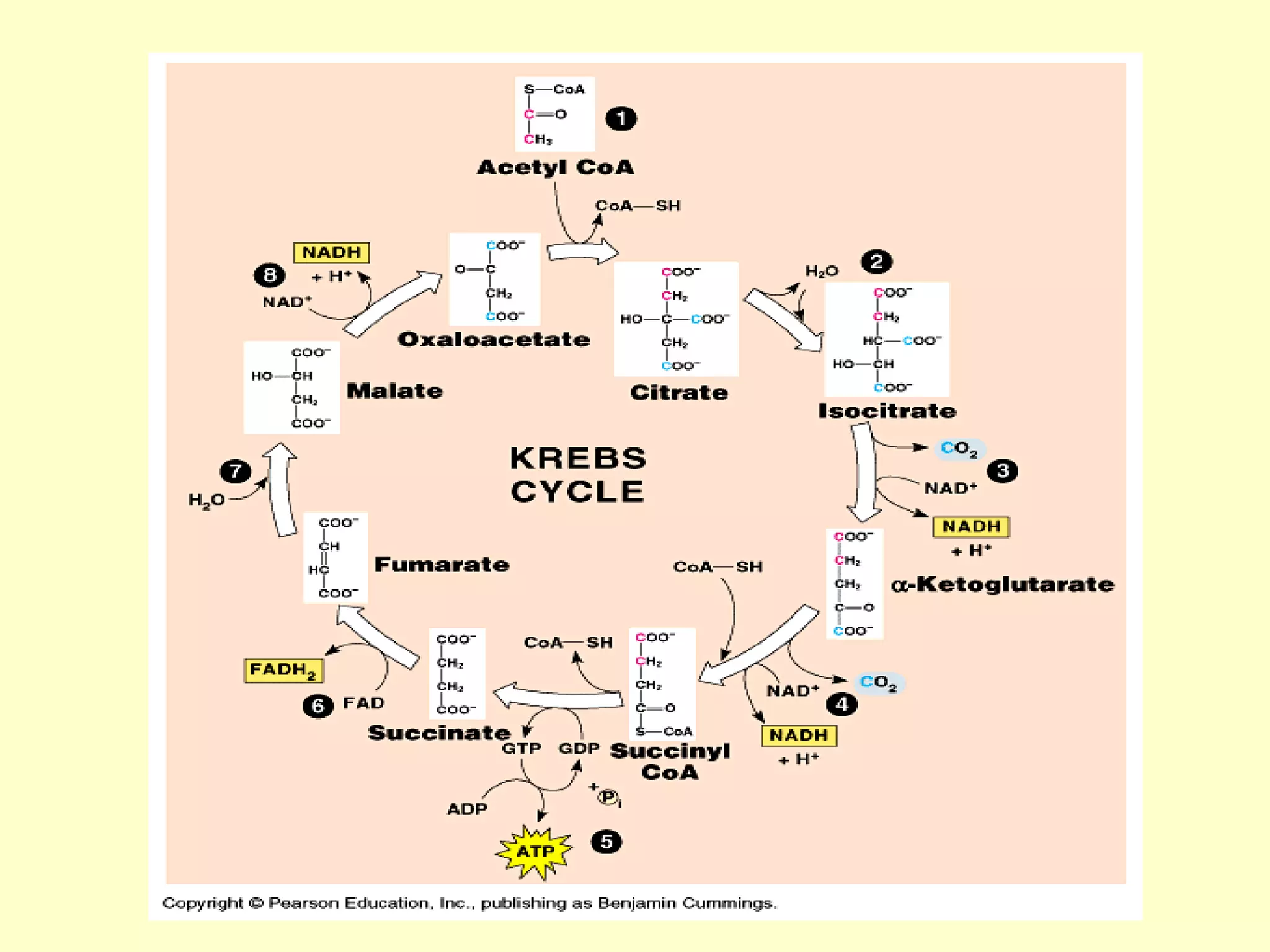
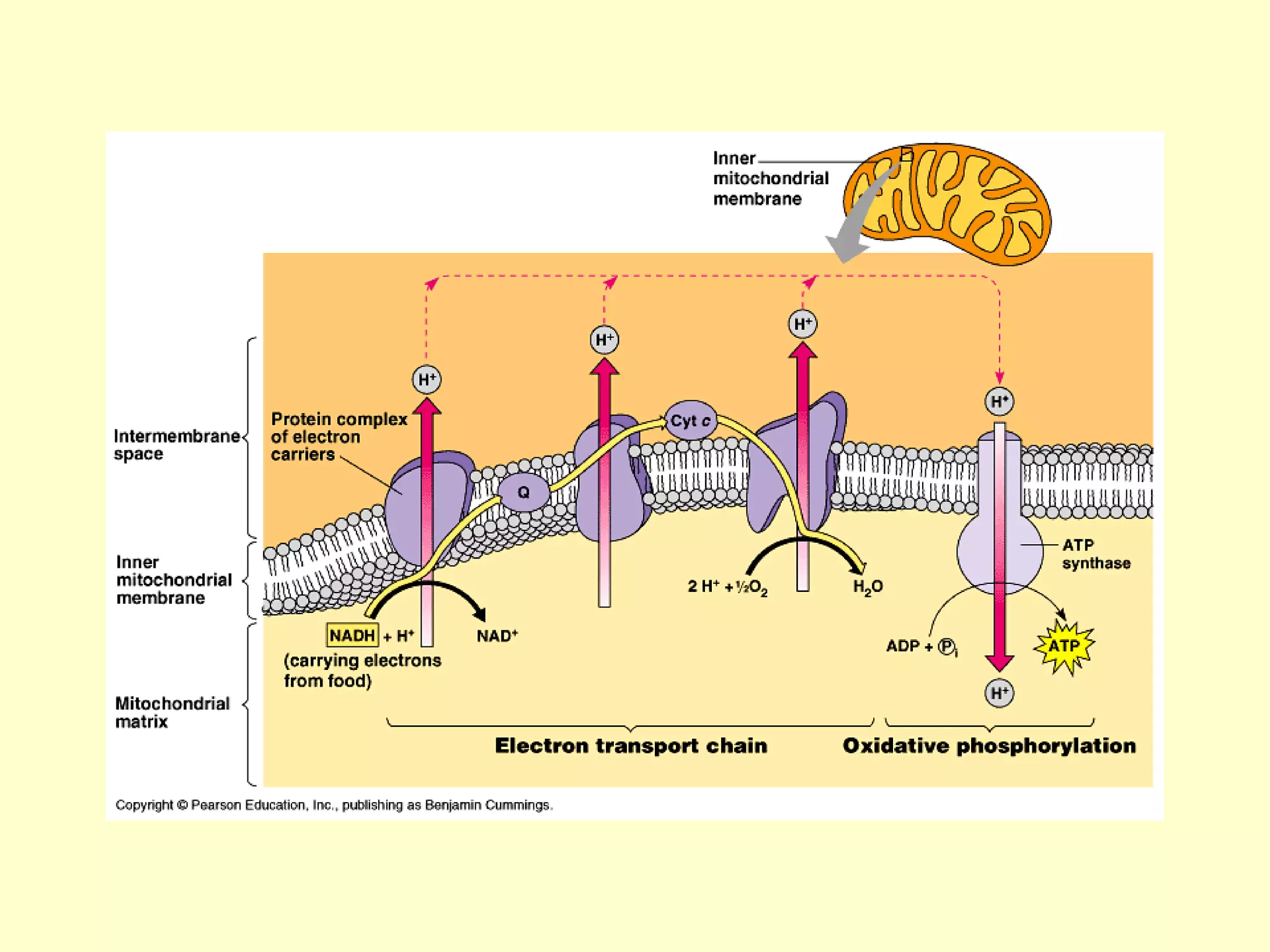
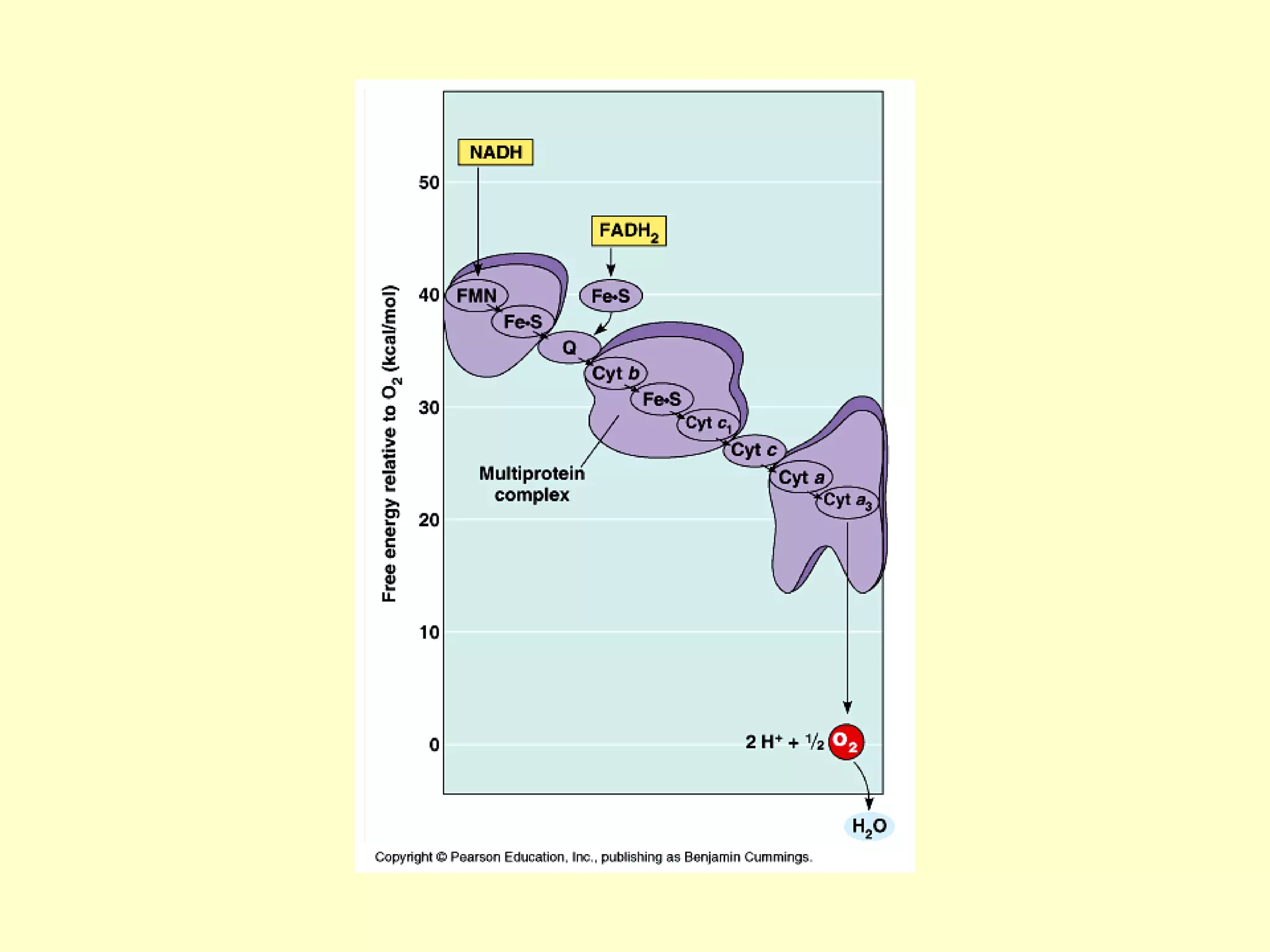
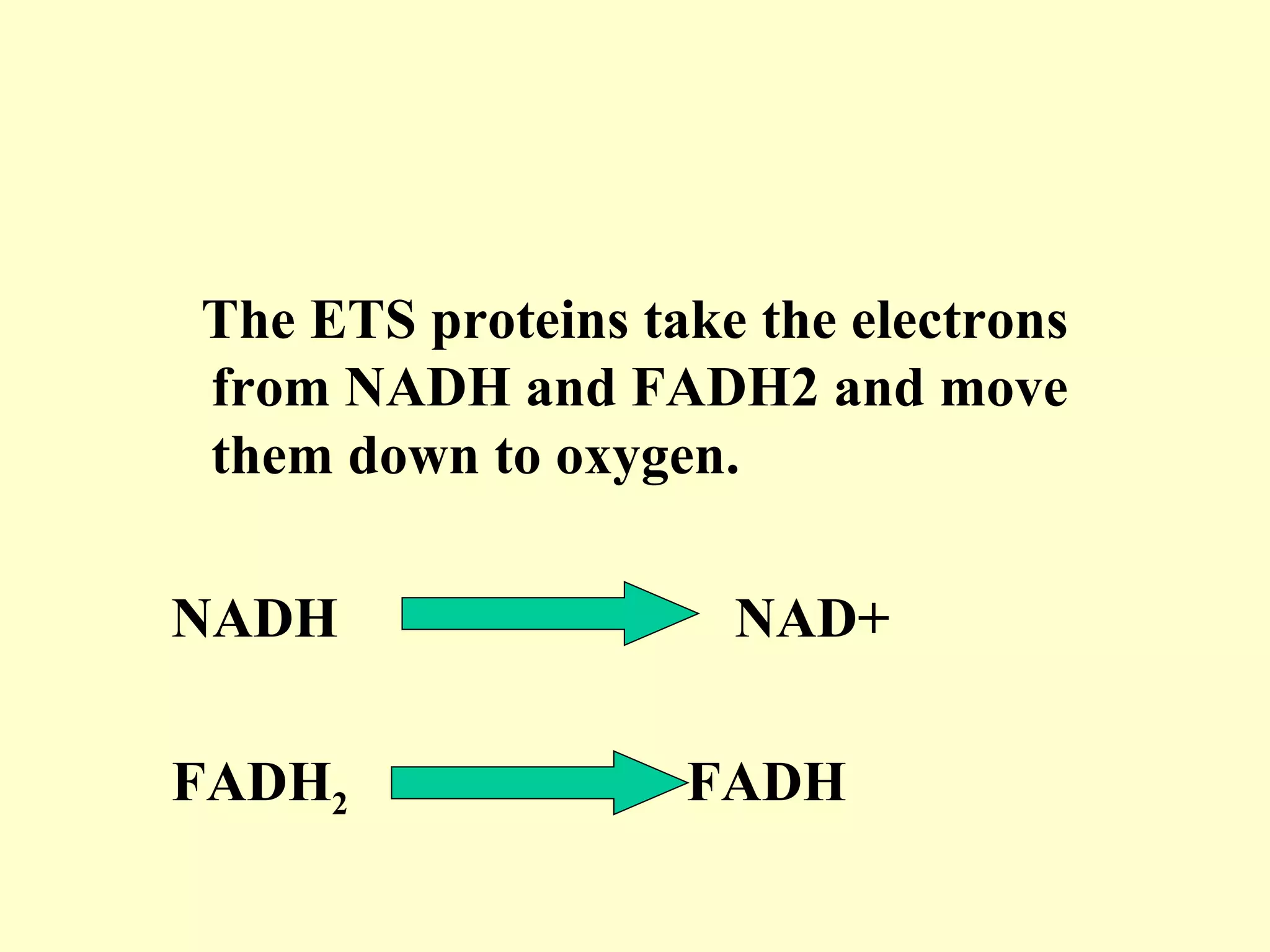
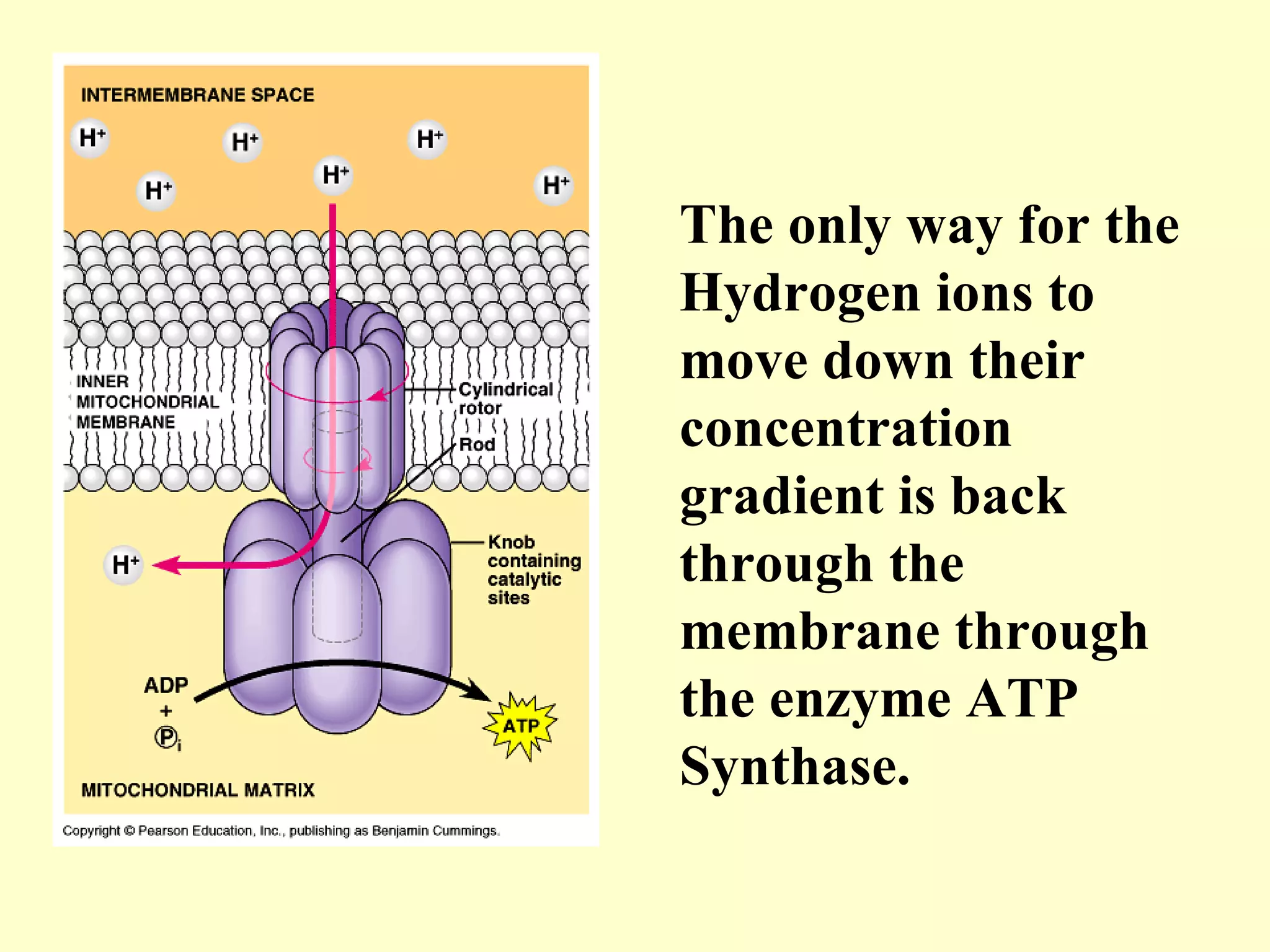
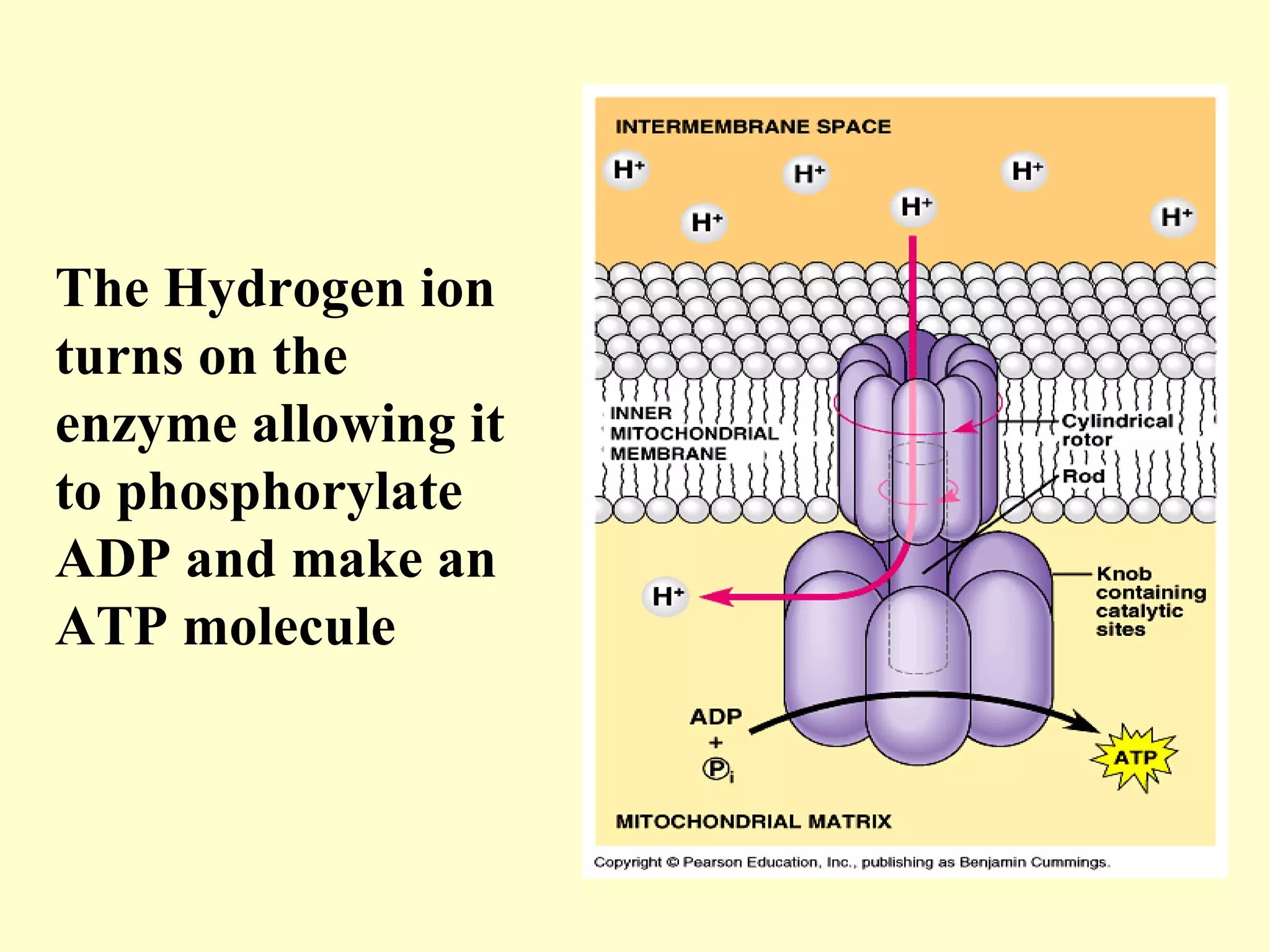
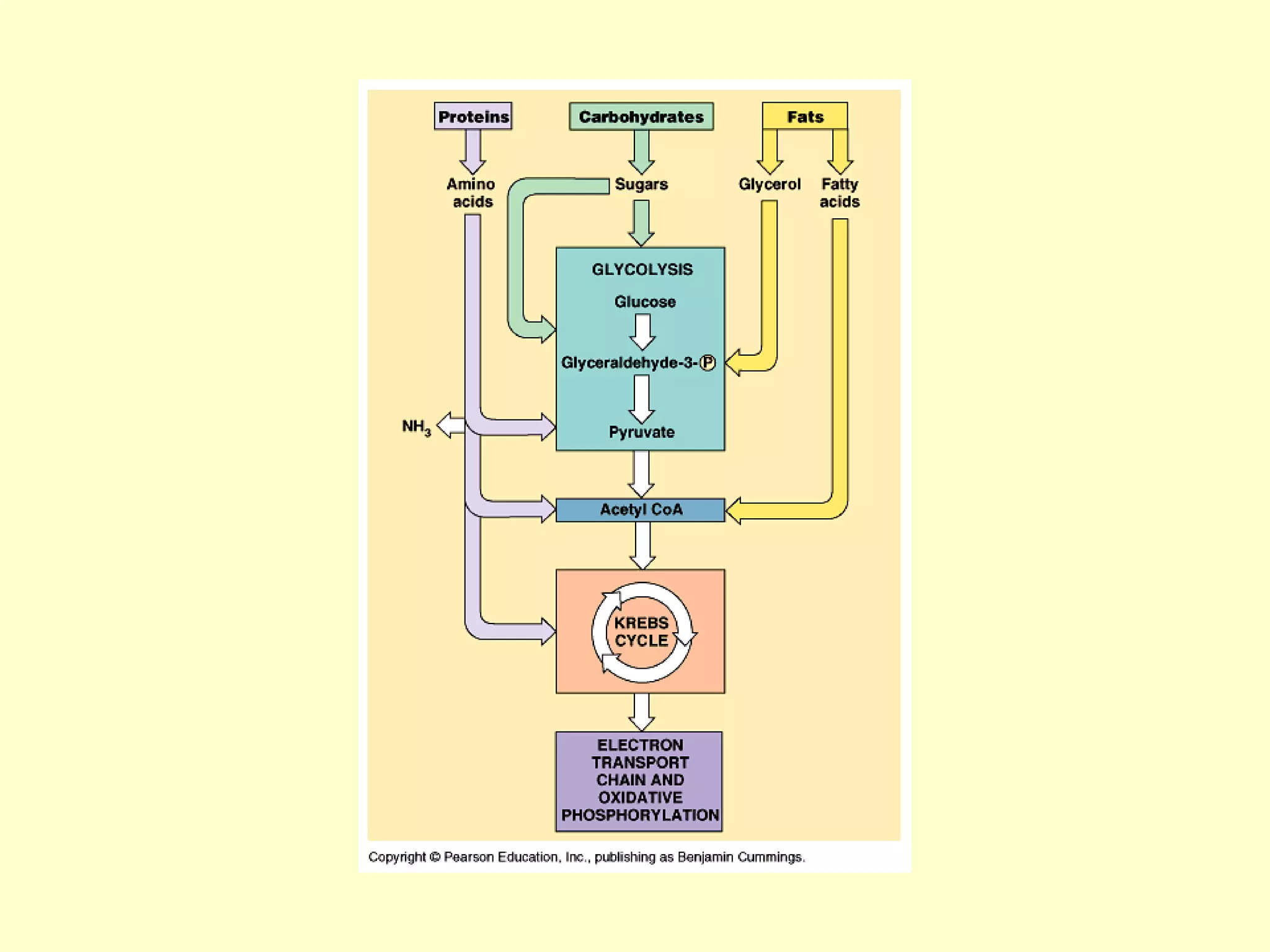
3) Aerobic respiration involves three main stages - glycolysis, the Krebs cycle in the mitochondria, and the electron transport chain - to fully break down glucose and produce large amounts of ATP through chemiosmosis.