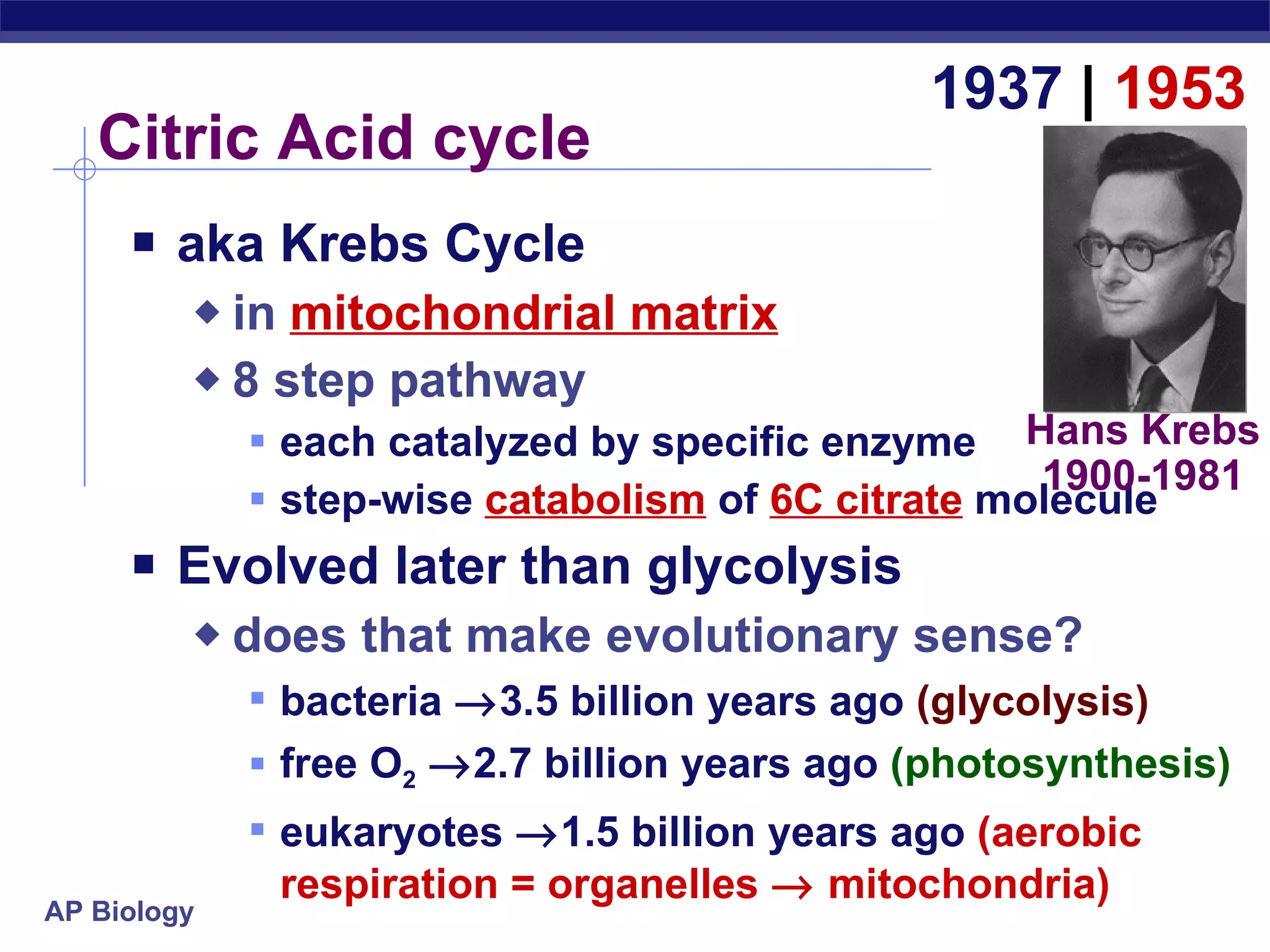
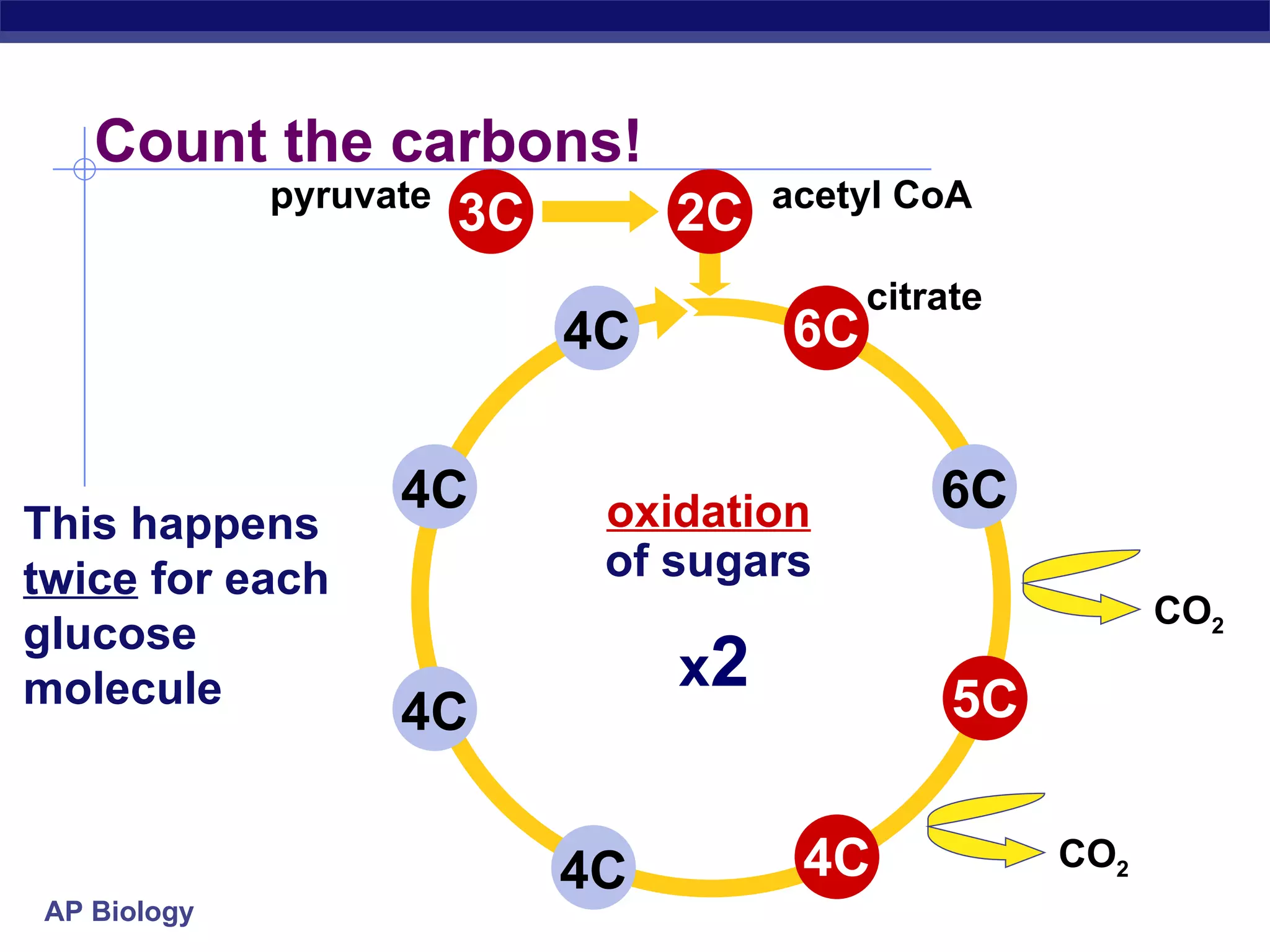
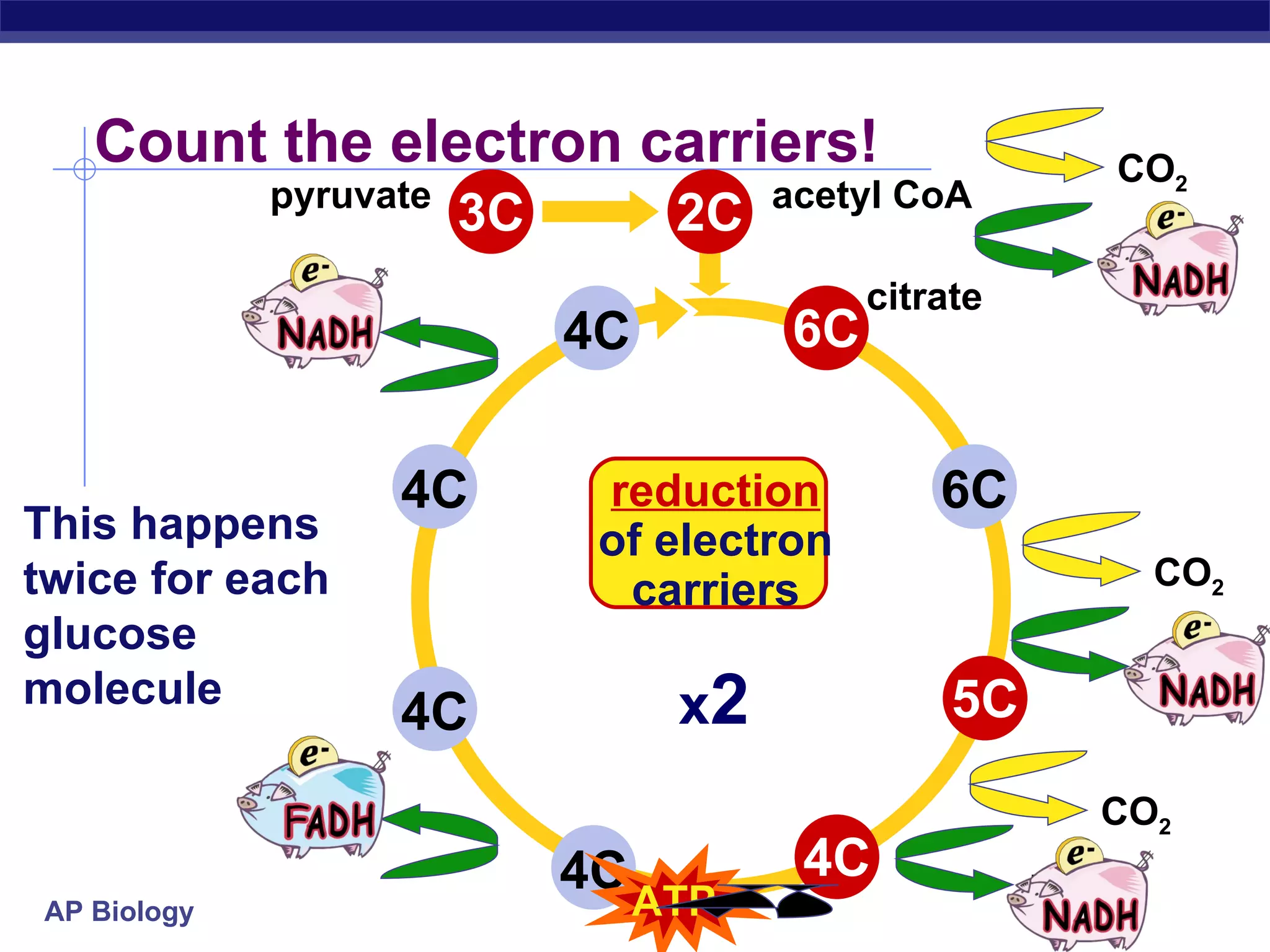
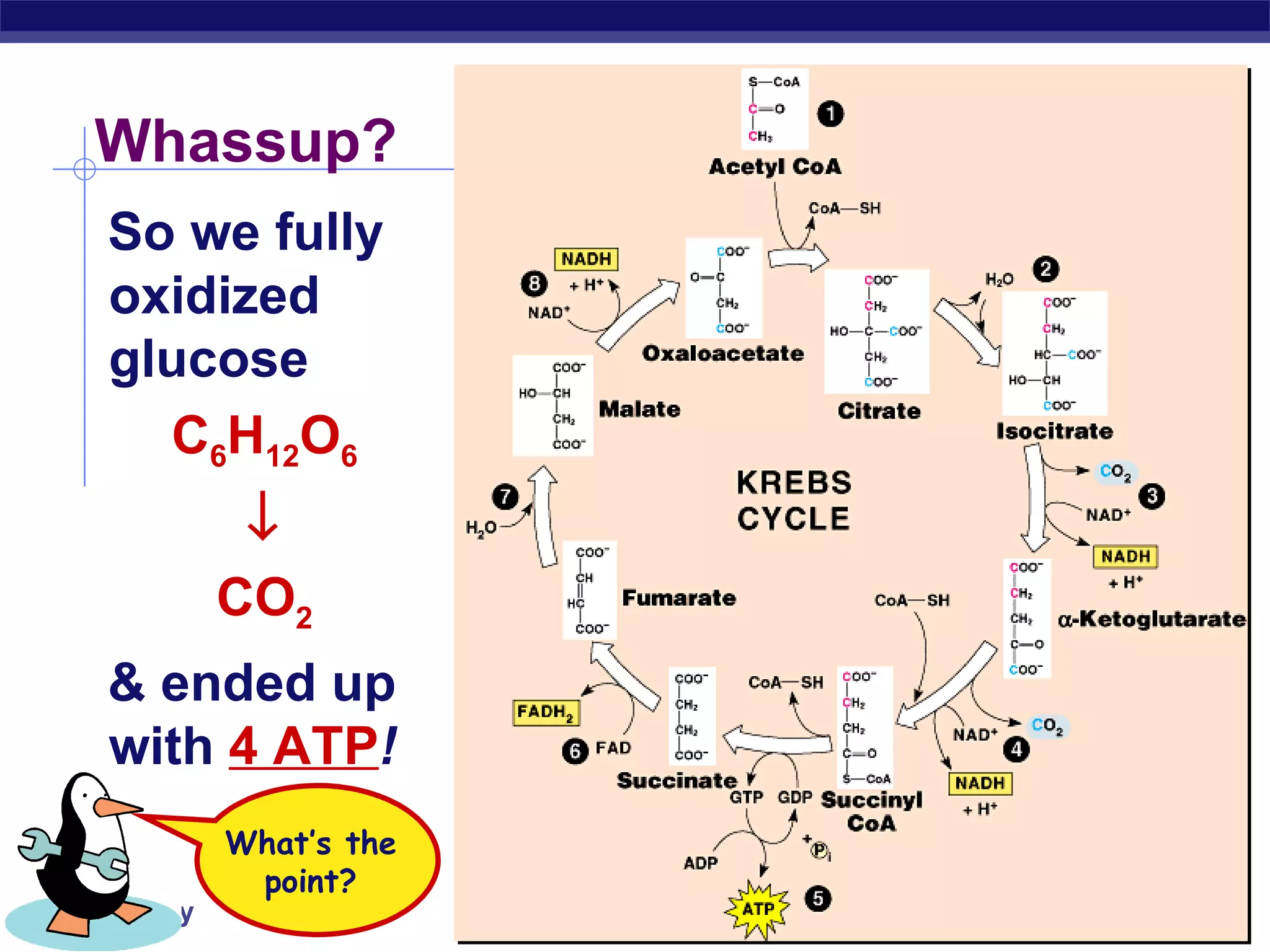
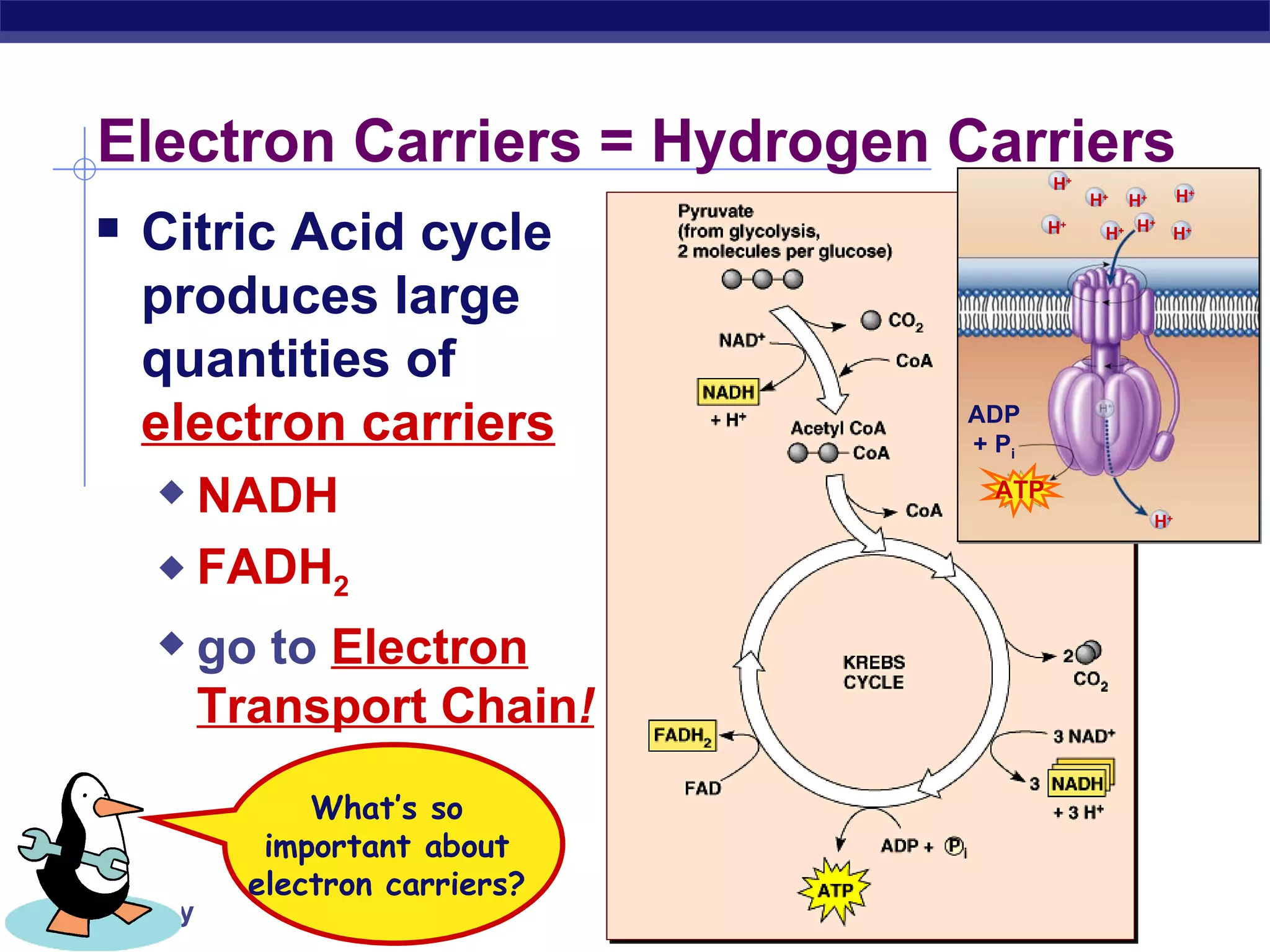
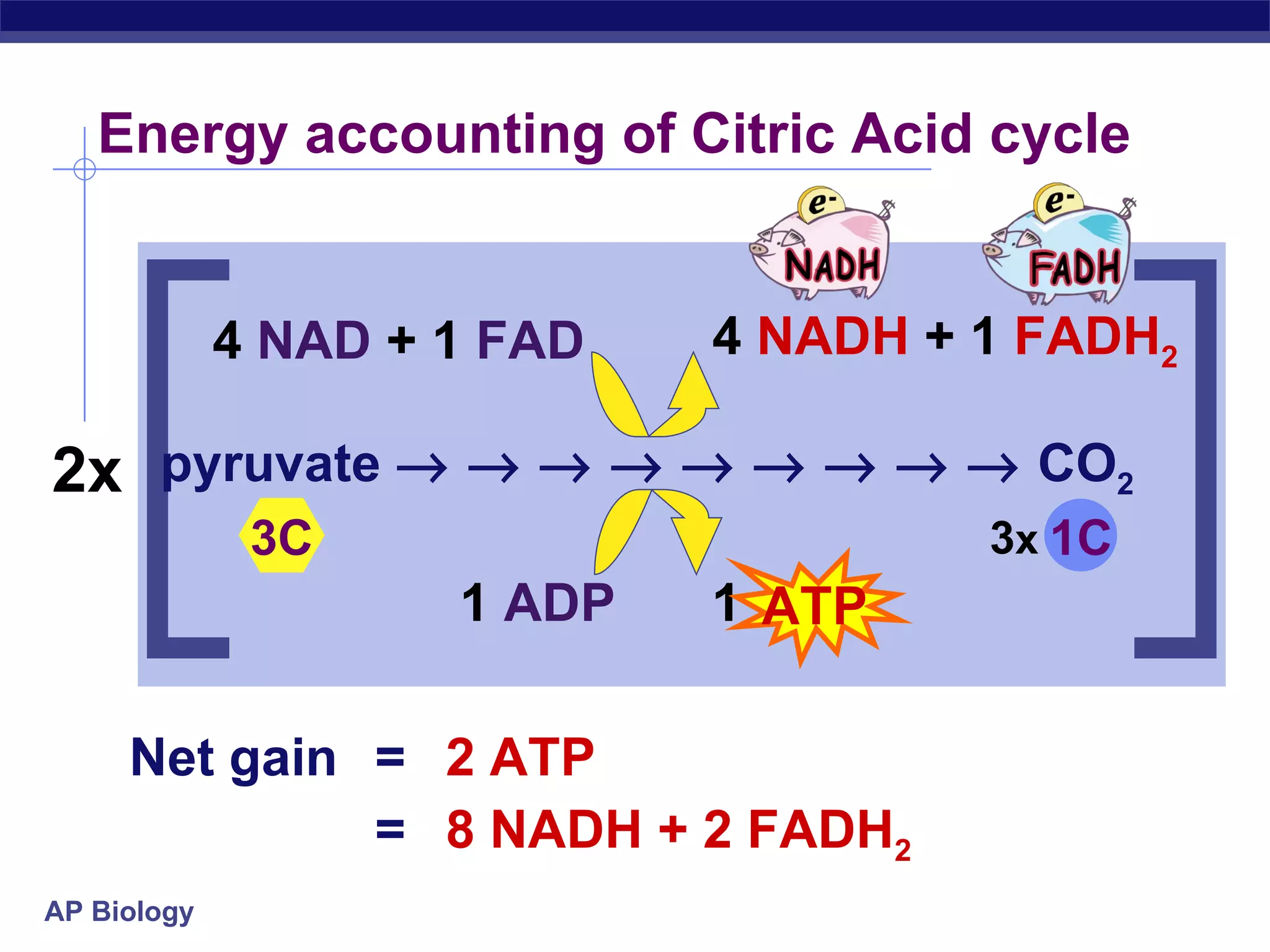
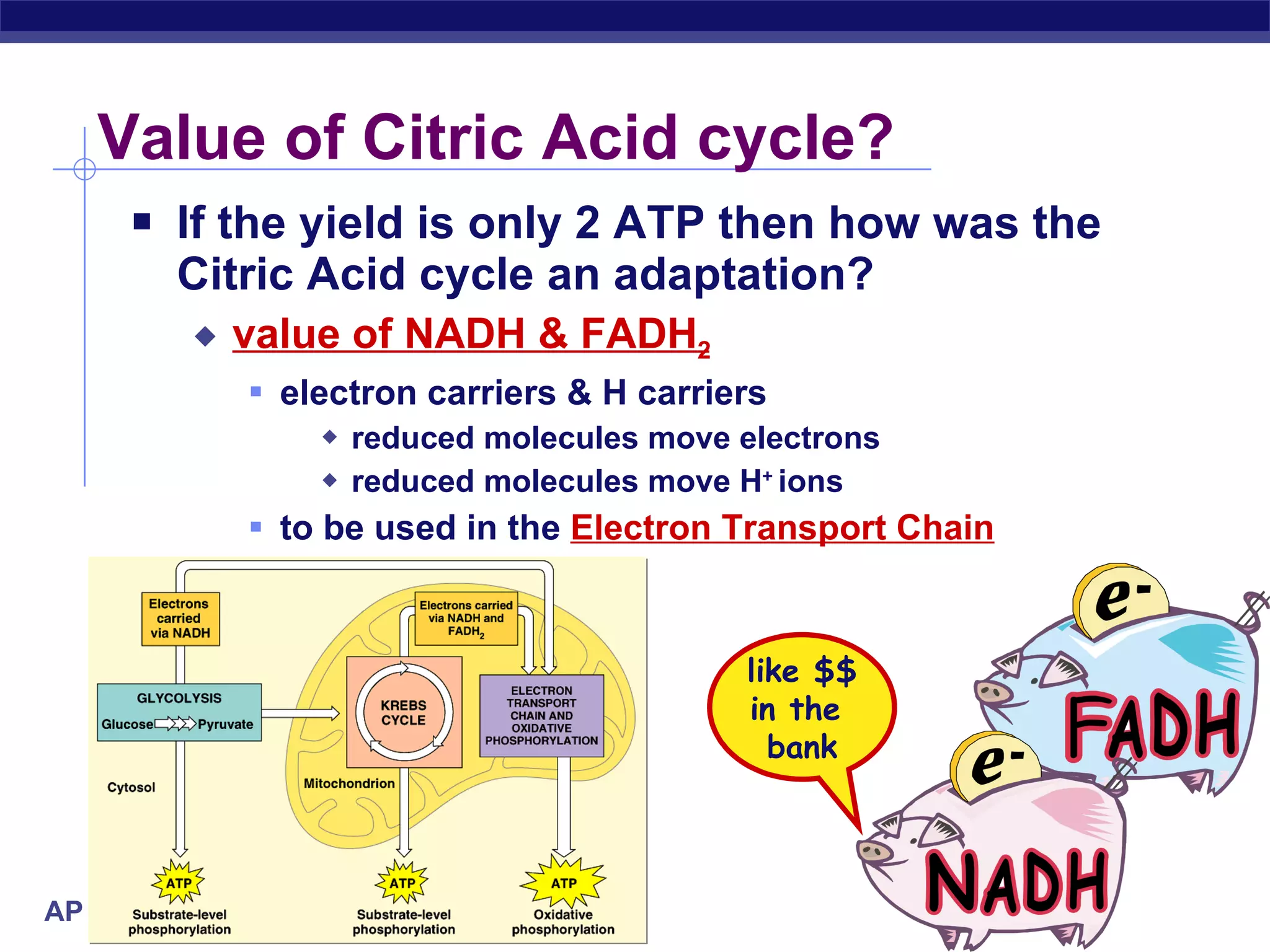
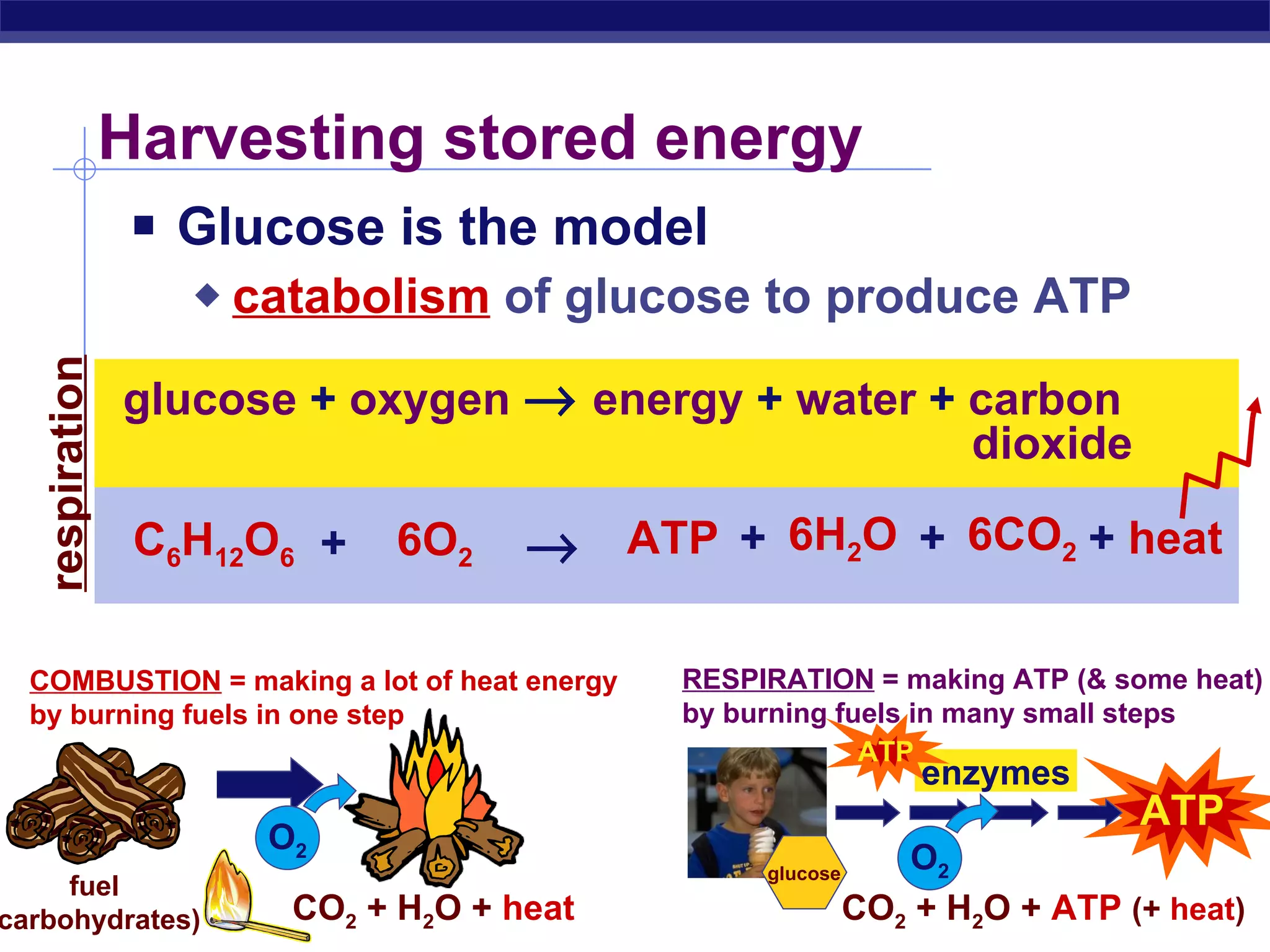
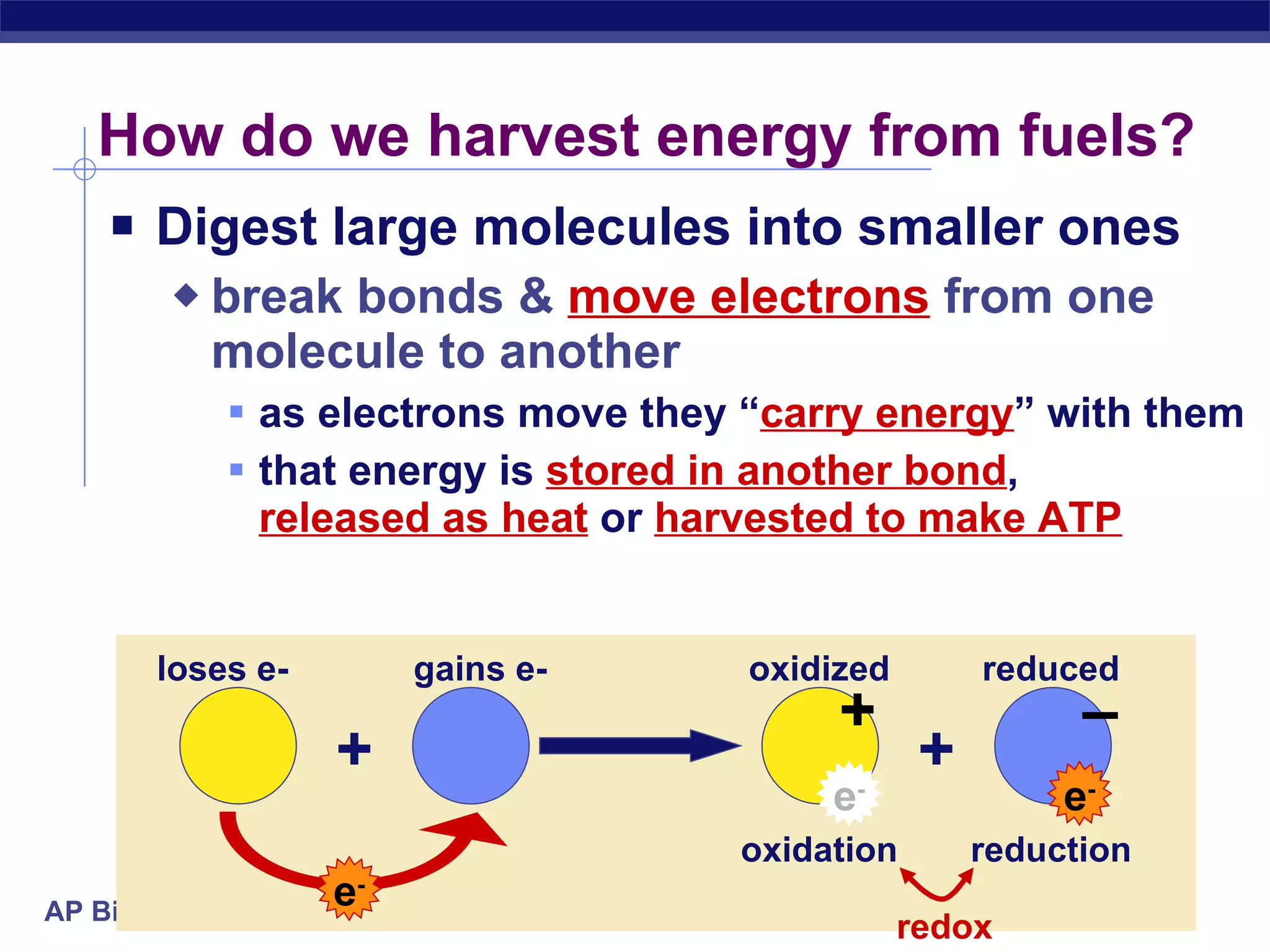
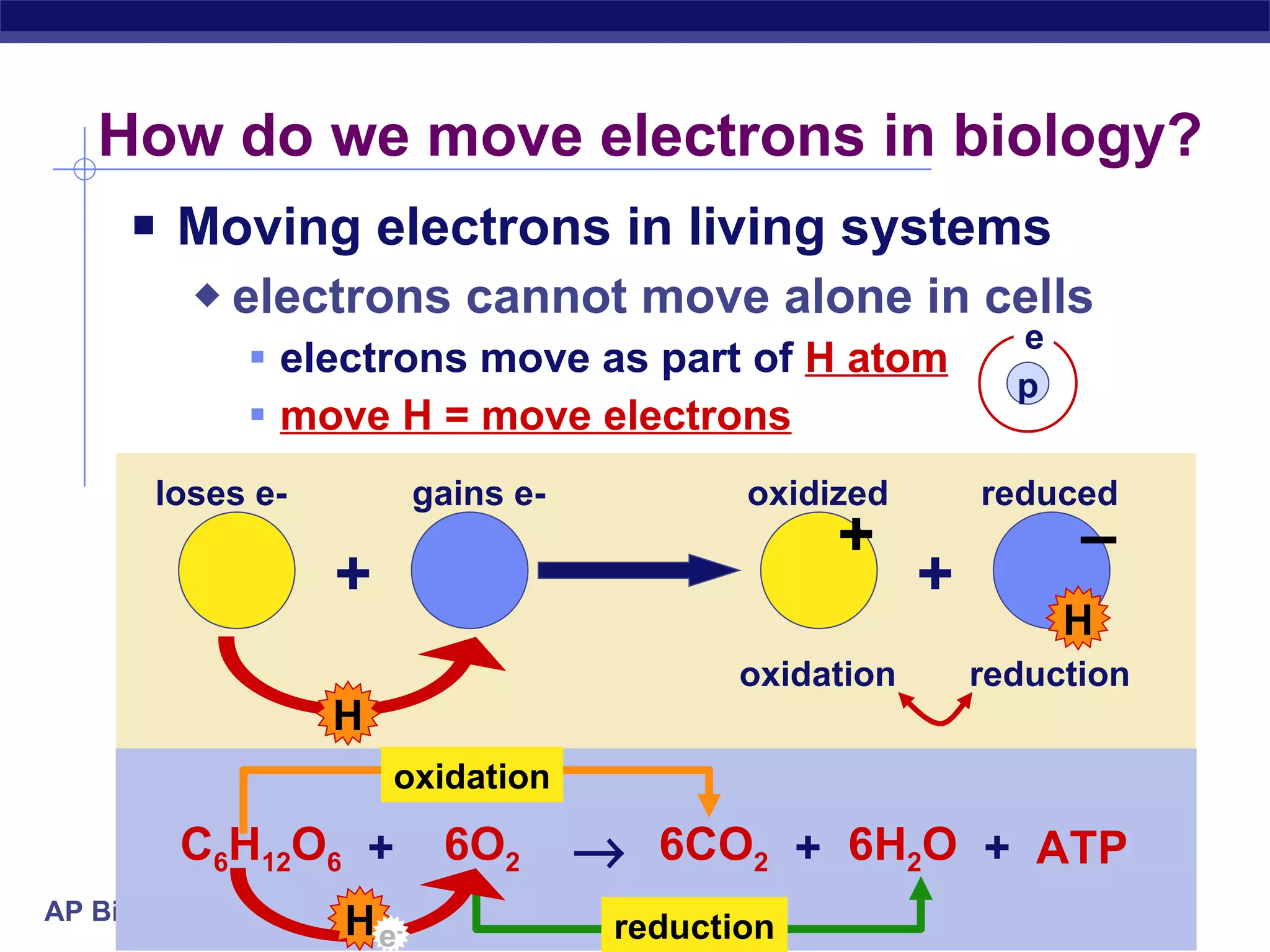
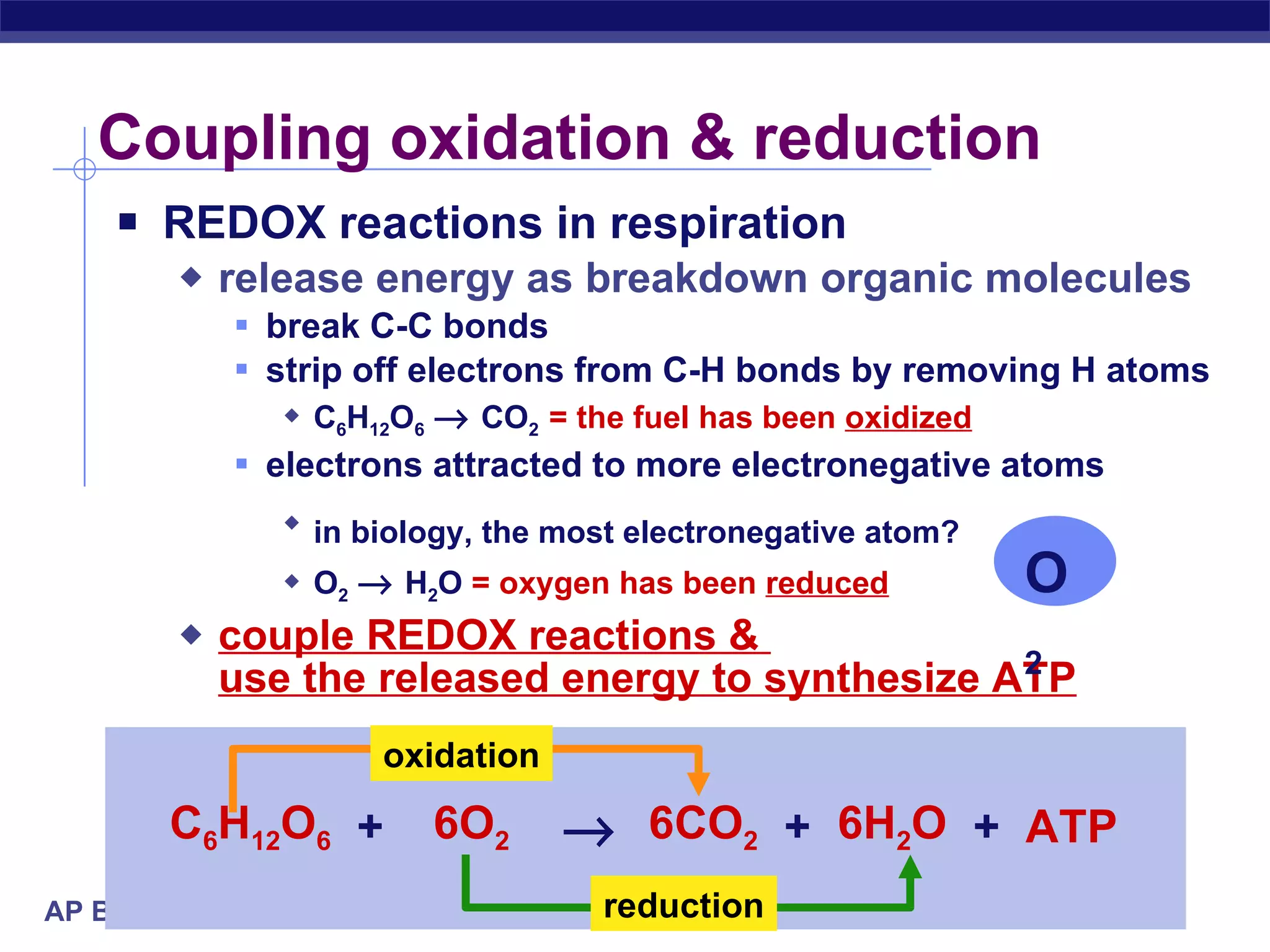
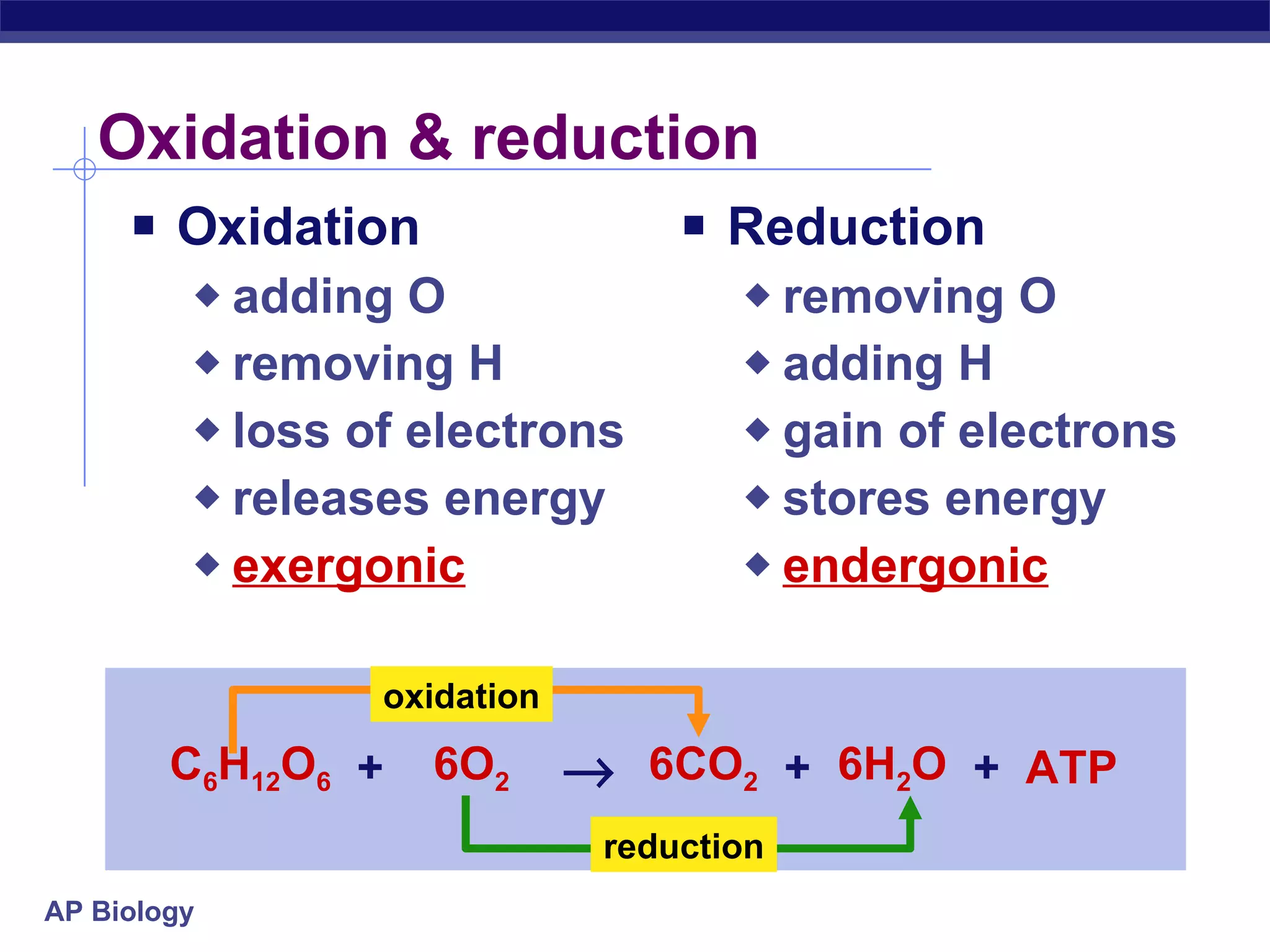
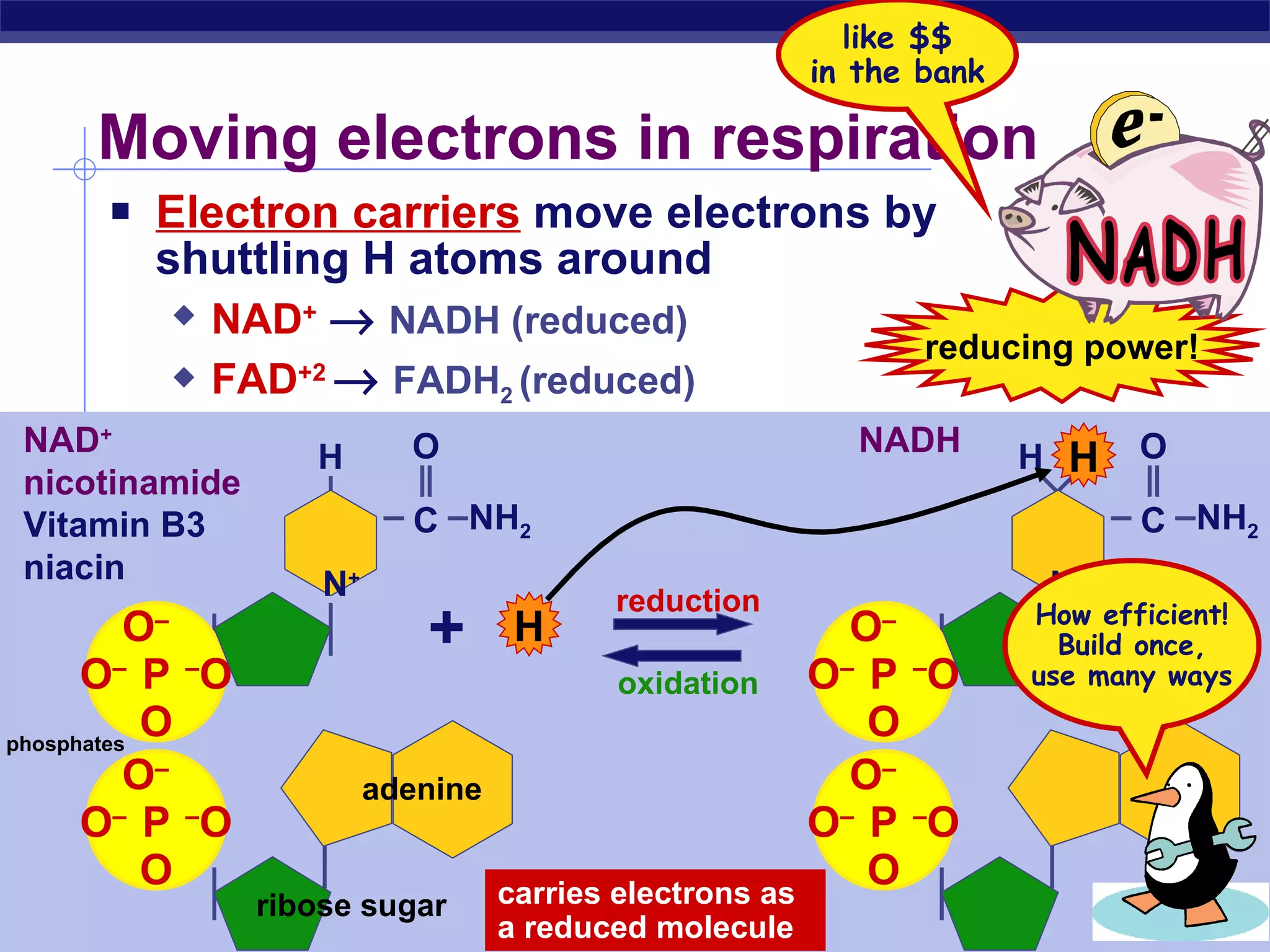
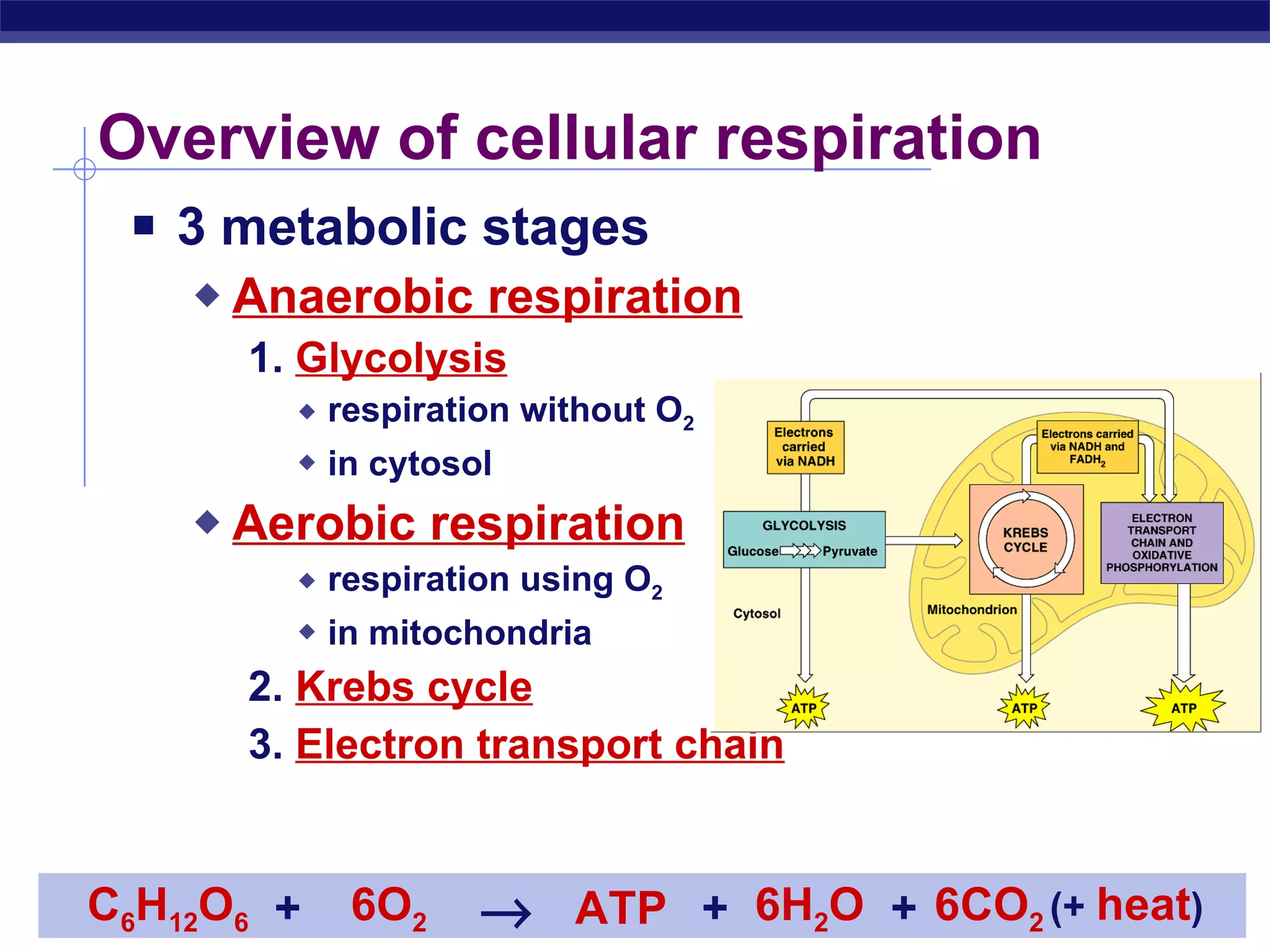
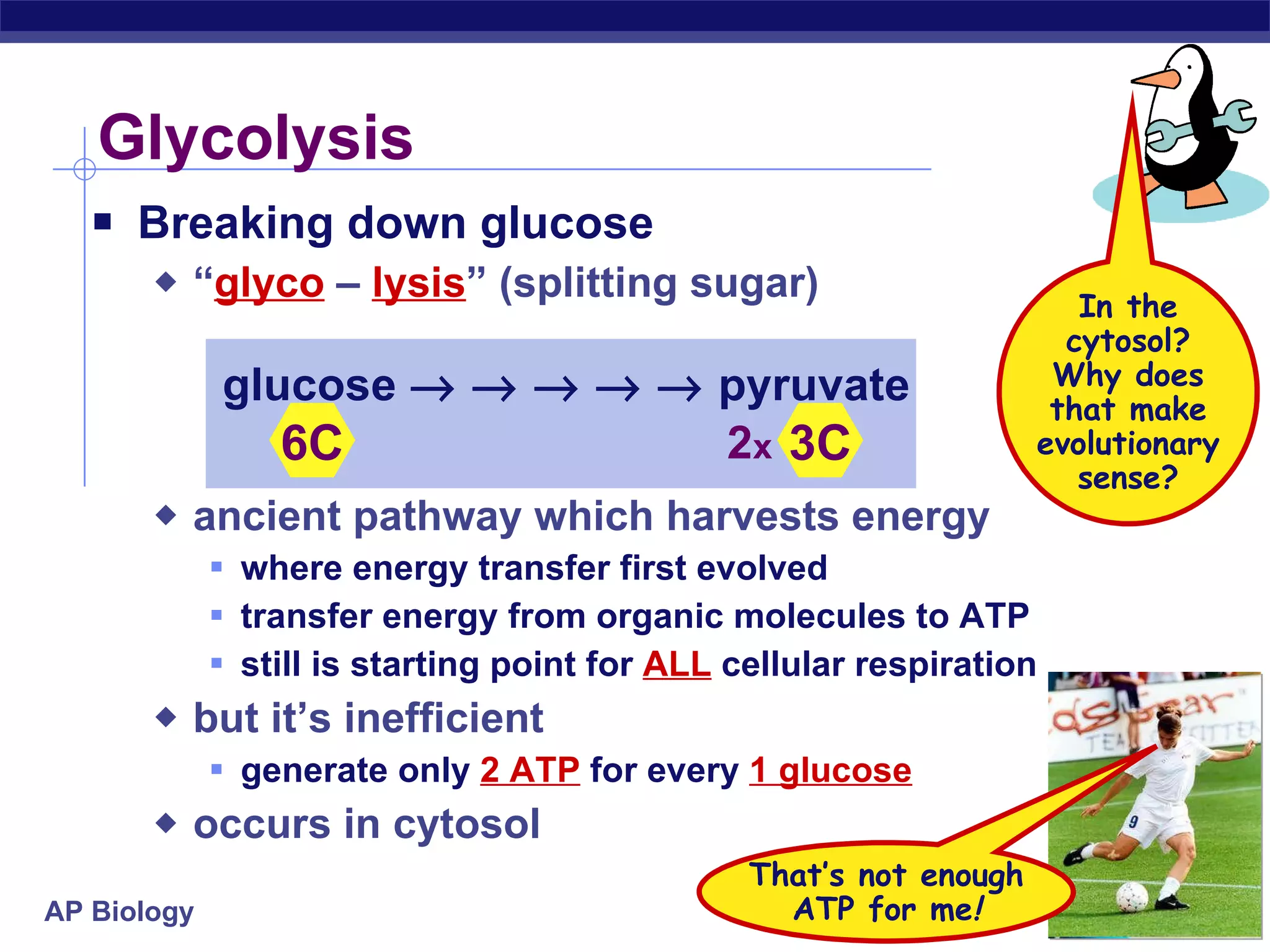
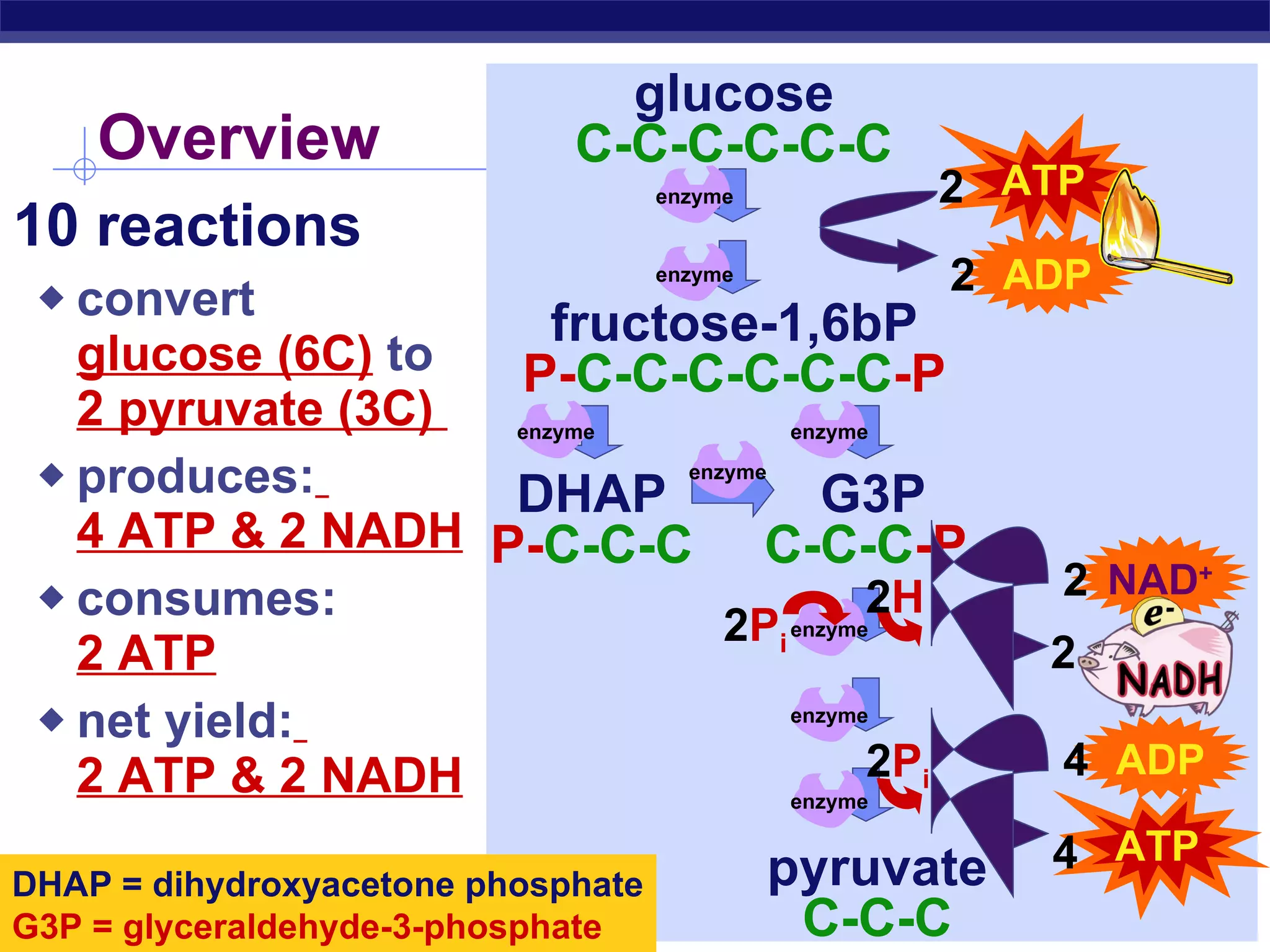
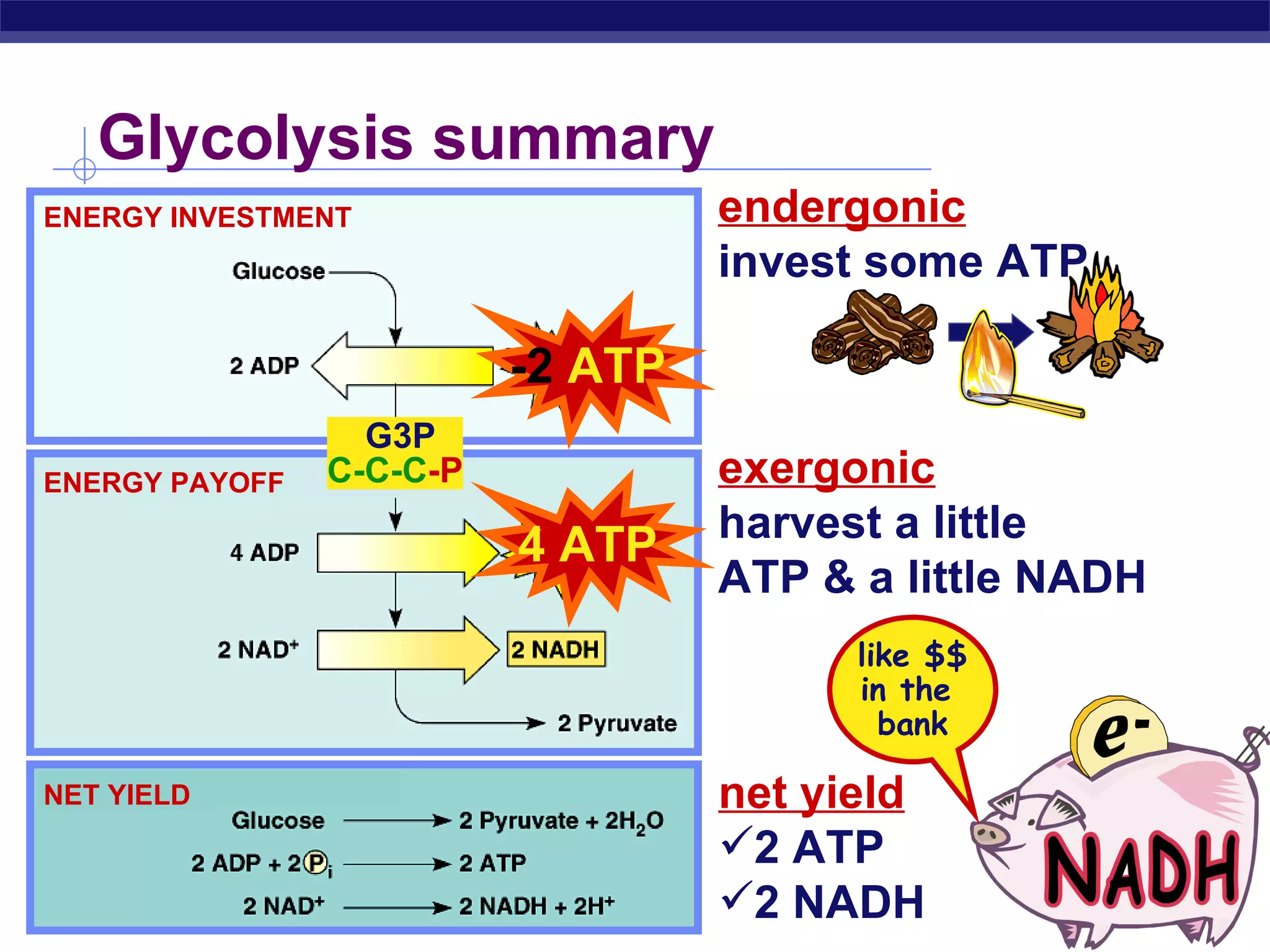
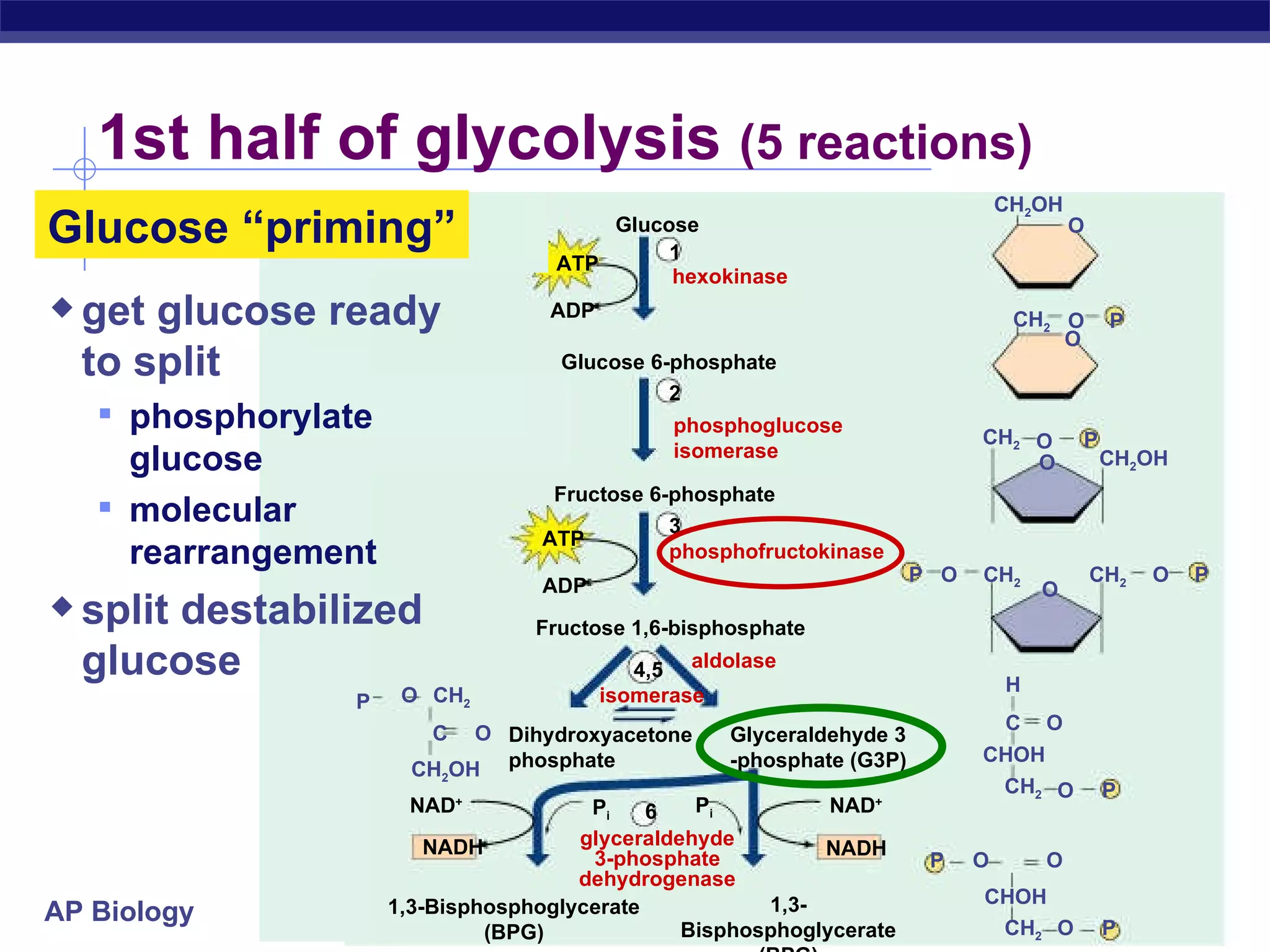
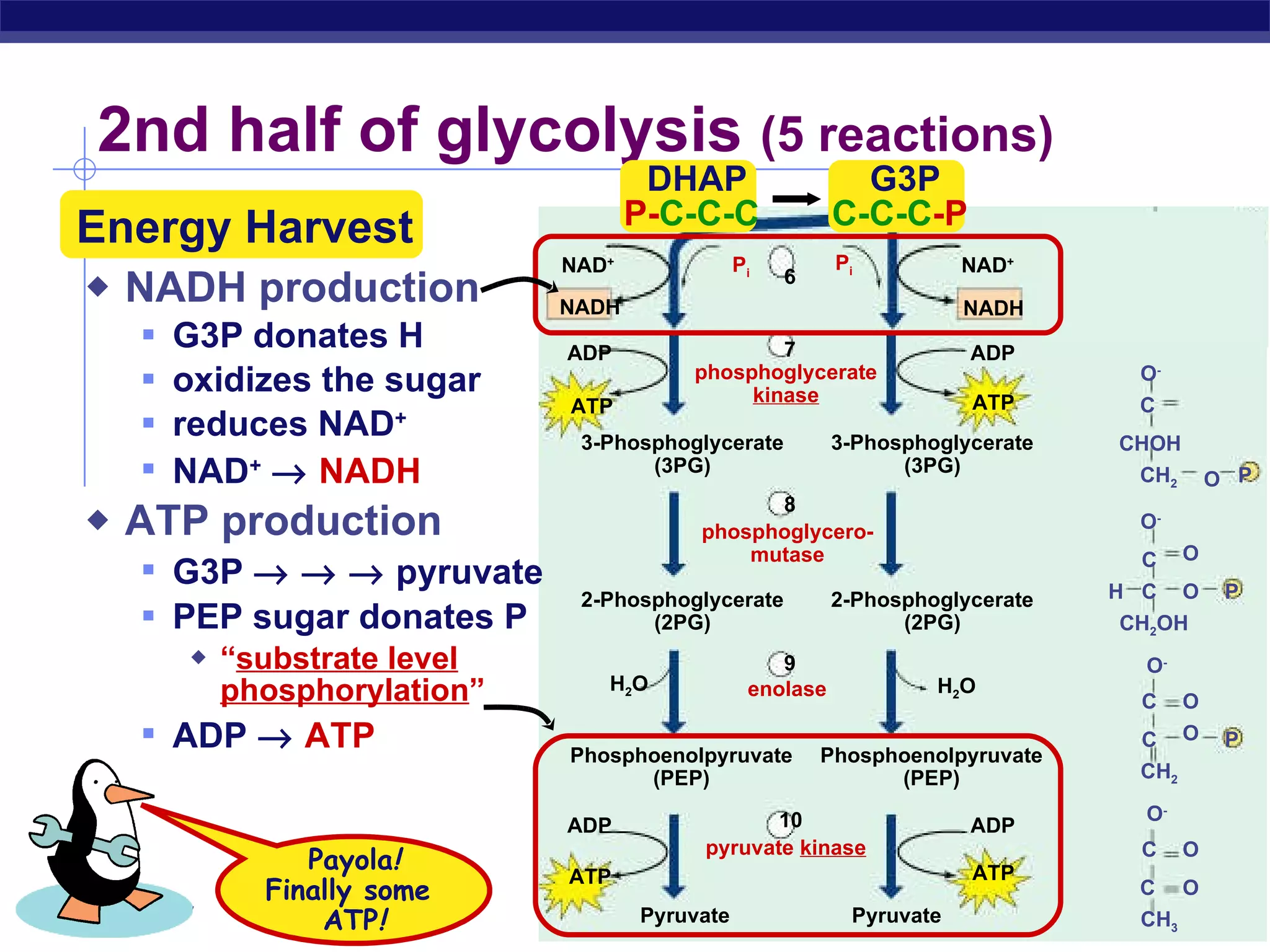
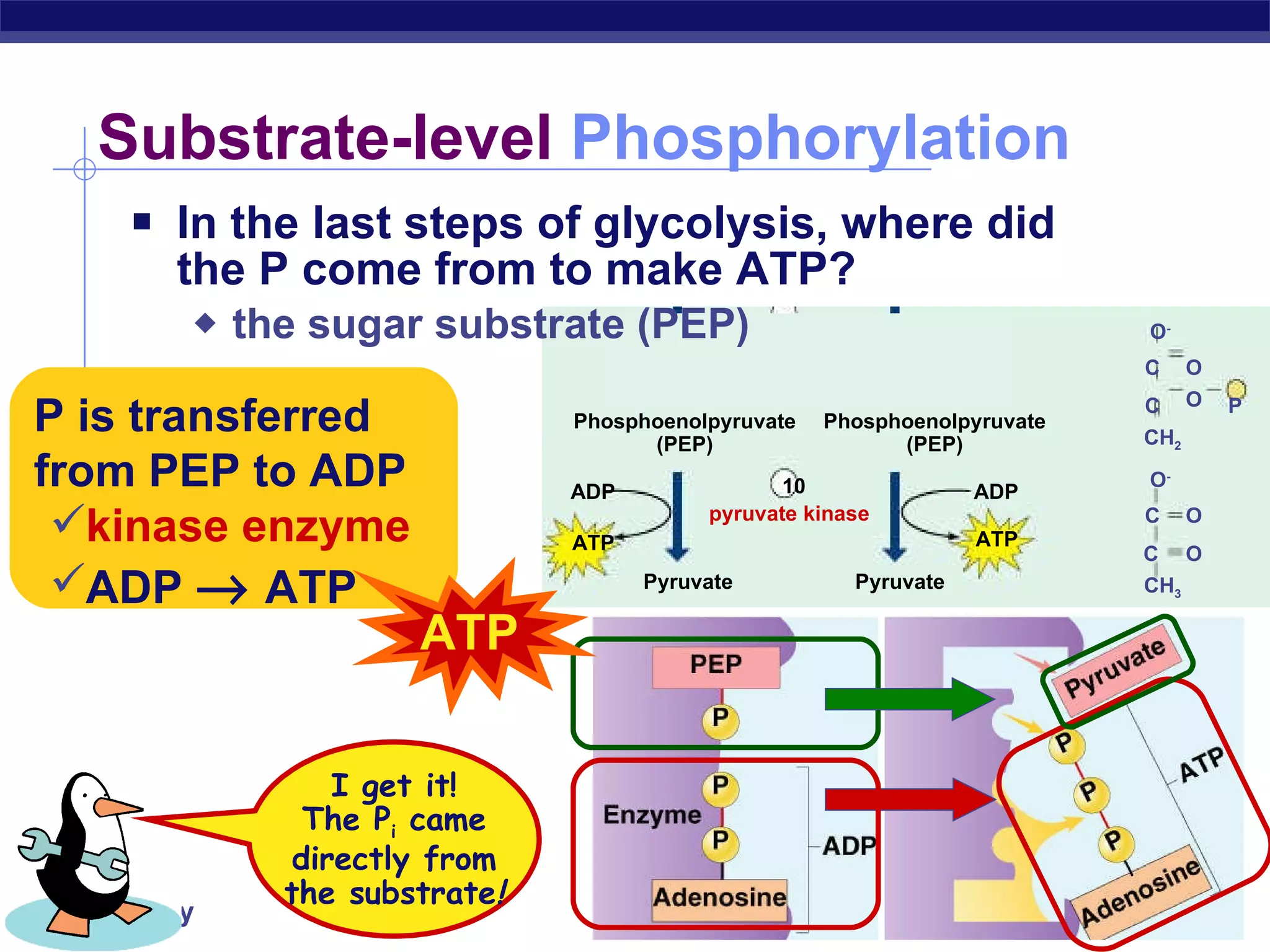
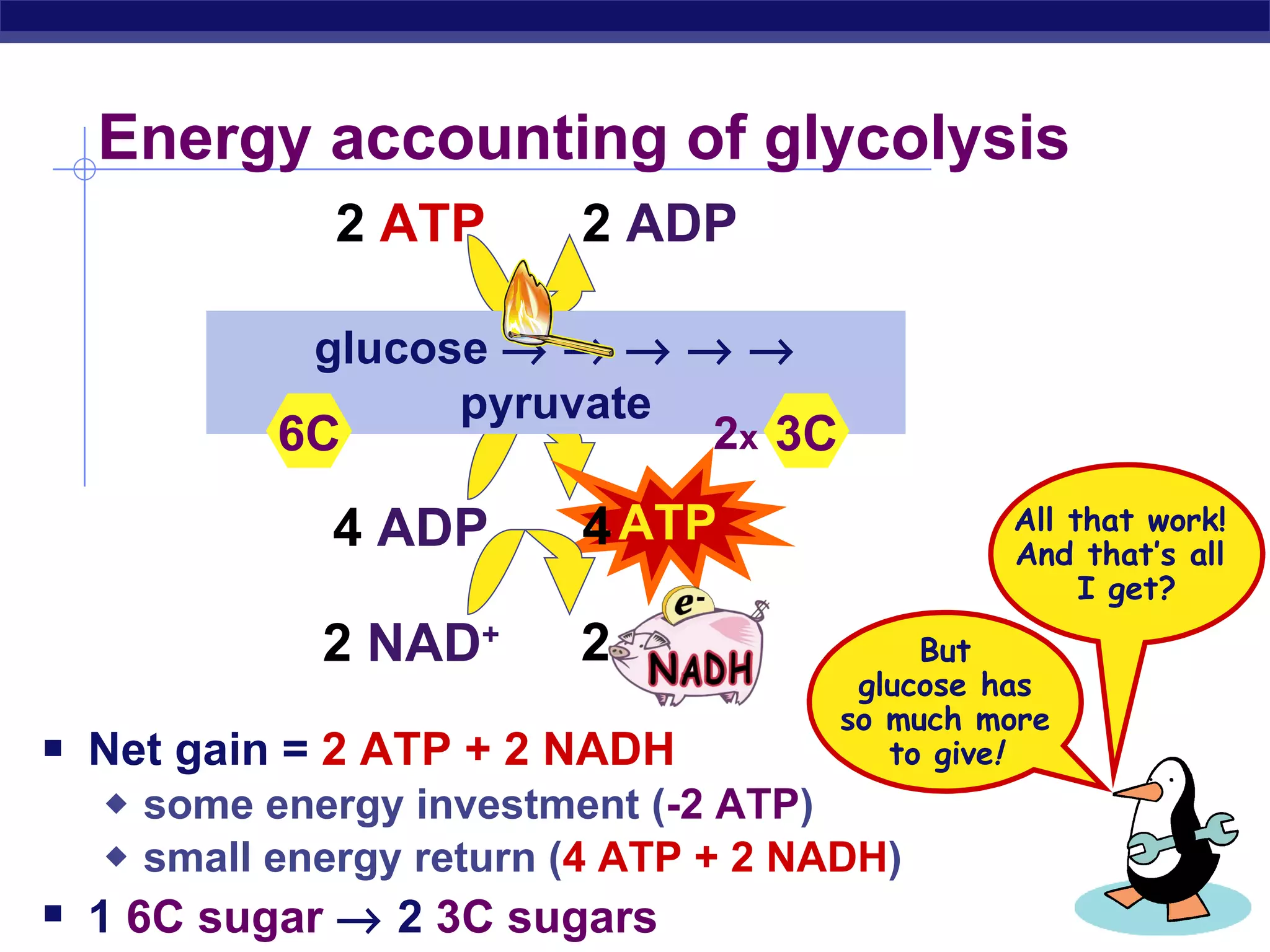
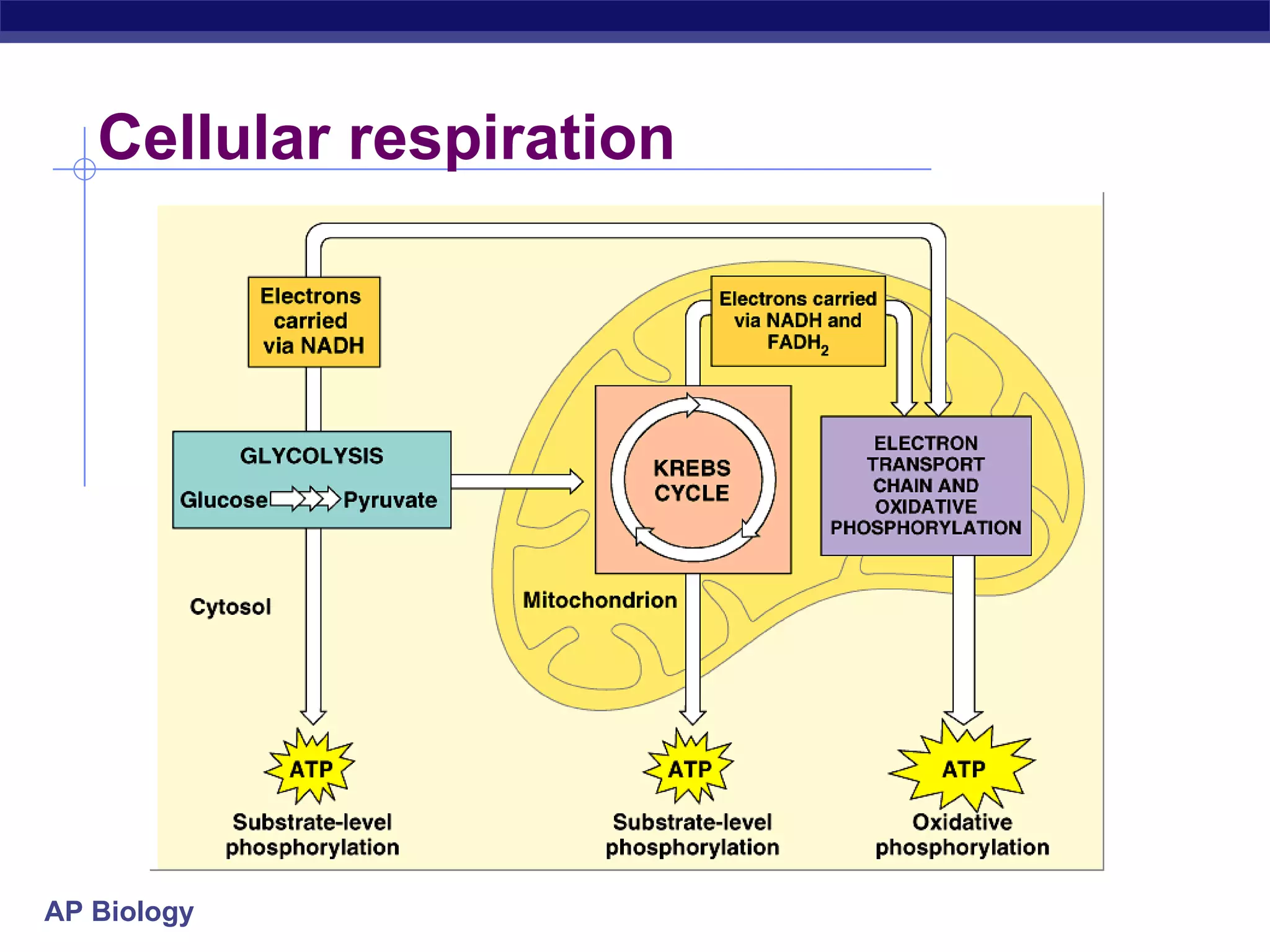
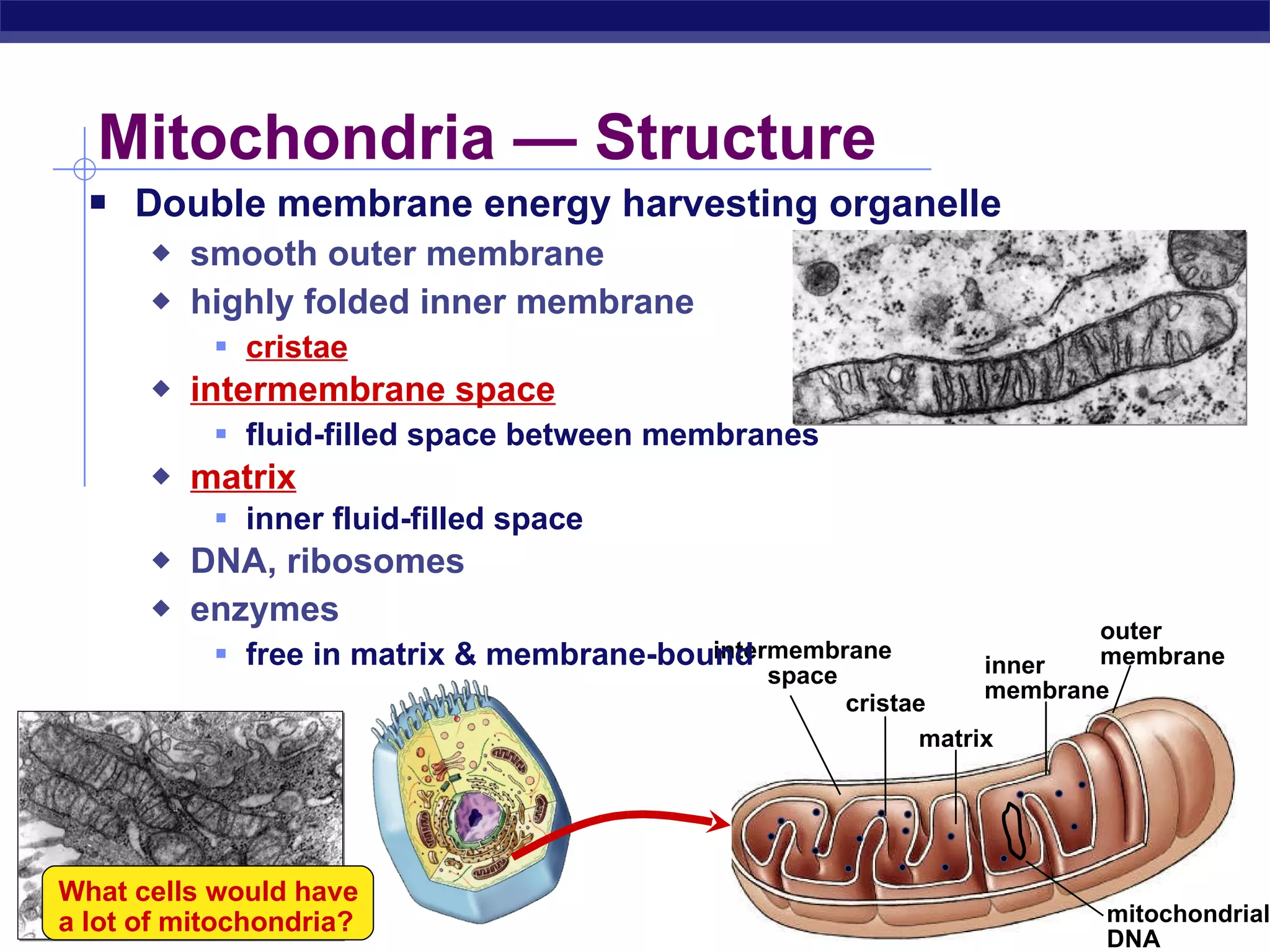
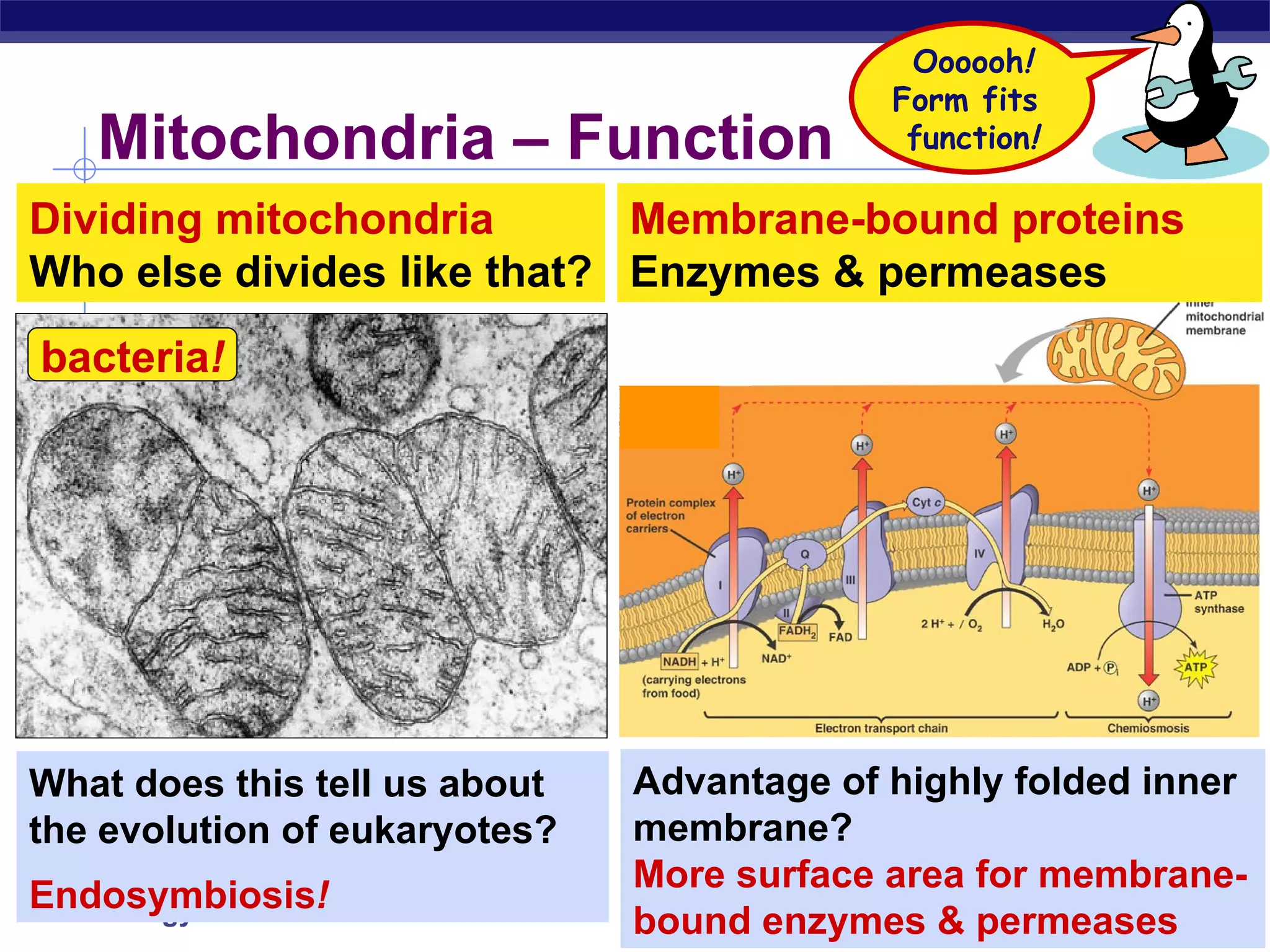
The document summarizes cellular respiration and the processes of glycolysis, the citric acid cycle, and the electron transport chain. Glycolysis breaks down glucose into pyruvate, producing 2 ATP and 2 NADH. The citric acid cycle further oxidizes pyruvate through a series of reactions, producing 8 NADH, 2 FADH2, and 2 ATP. The electron transport chain uses the electron carriers NADH and FADH2 to produce large amounts of ATP through oxidative phosphorylation.


![Oxidation of pyruvate Pyruvate enters mitochondrial matrix 3 step oxidation process releases 2 CO 2 (count the carbons!) reduces 2 NAD 2 NADH (moves e - ) produces 2 acetyl CoA Acetyl CoA enters Krebs cycle 3C 2C 1C Where does the CO 2 go? Exhale ! pyruvate acetyl CoA + CO 2 NAD [ 2x ]](https://image.slidesharecdn.com/chapter91-32-091022053234-phpapp01/75/Chapter-9-23-2048.jpg)
![Pyruvate oxidized to Acetyl CoA Yield = 2C sugar + NADH + CO 2 reduction oxidation Coenzyme A Pyruvate Acetyl CoA C-C-C C-C CO 2 NAD + 2 x [ ]](https://image.slidesharecdn.com/chapter91-32-091022053234-phpapp01/75/Chapter-9-24-2048.jpg)