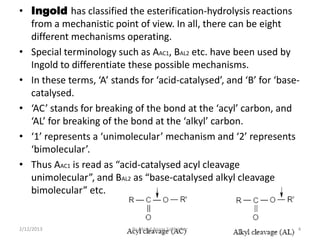
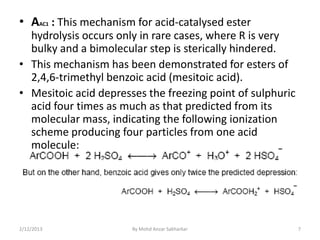
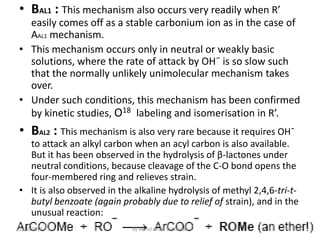
The document discusses various mechanisms for ester hydrolysis reactions. It describes 8 possible mechanisms - AAC1, AAC2, AAL1, etc. - that differentiate between acid-catalyzed and base-catalyzed reactions, and whether the bond breaks at the acyl or alkyl carbon. The most common mechanisms are AAC2 for acid hydrolysis, involving a tetrahedral intermediate, and BAC2 for base hydrolysis, also proceeding through a tetrahedral intermediate. Rare mechanisms like AAC1 and BAL1 can occur for certain esters when the leaving group easily forms a stable carbonium ion.