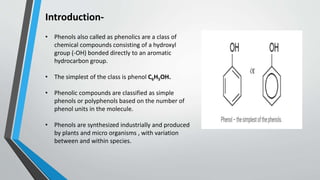
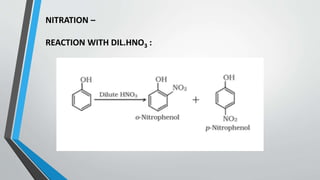
This document provides an overview of phenols, including their preparation from haloarenes, benzene sulphonic acid, diazonium salts, and cumene. It describes the physical properties of phenols, such as their higher boiling points compared to hydrocarbons due to hydrogen bonding. Several reactions of phenols are also outlined, including Kolbe's reaction, Reimer-Tiemann reaction, Fries rearrangement, acetylation, nitration, halogenation, and their reaction with bromine water. Finally, the document lists some common uses of phenols as precursors for plastics, drugs, disinfectants, and in molecular biology techniques.