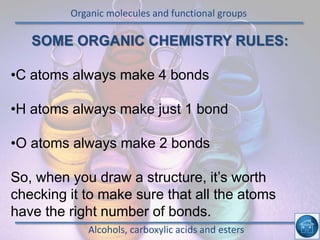
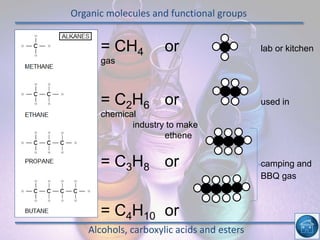
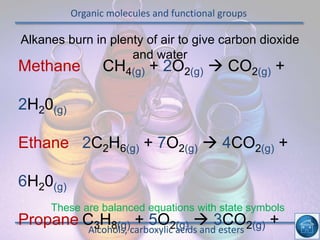
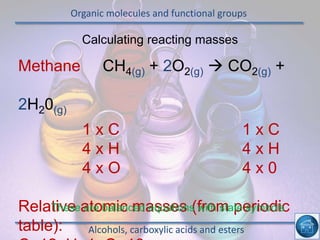
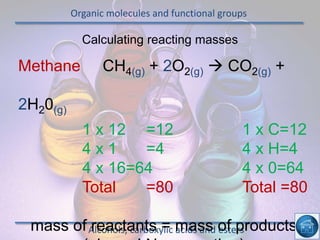
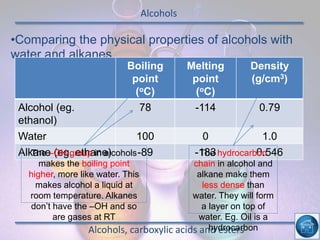
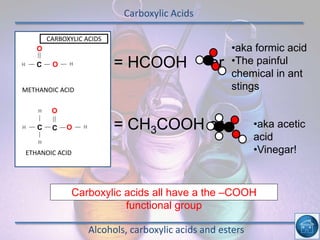
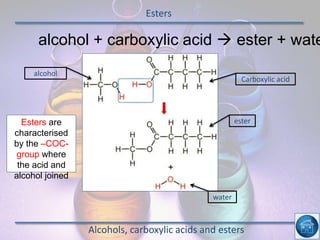
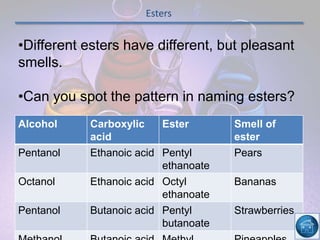
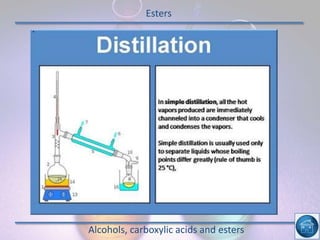
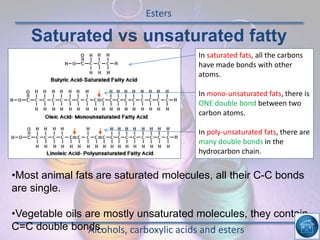
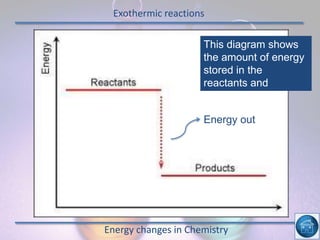
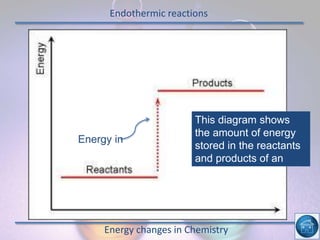
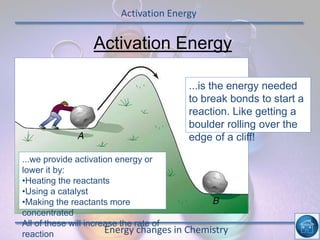
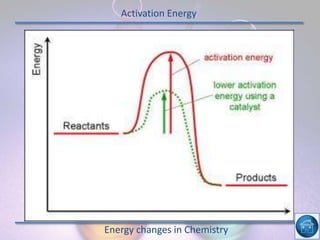
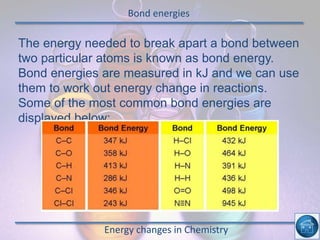
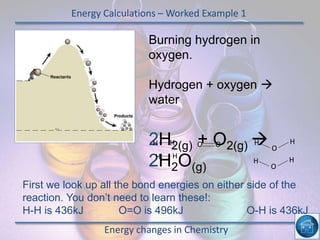
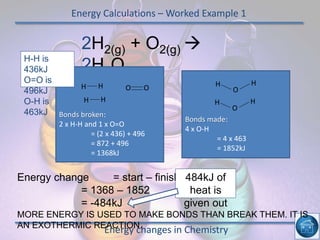
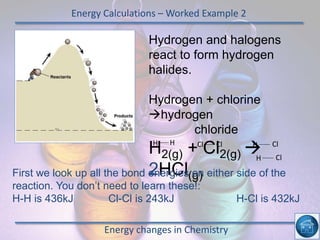
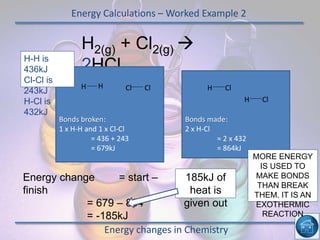
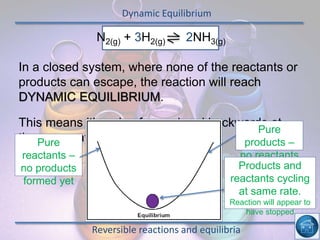
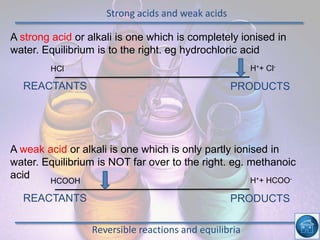
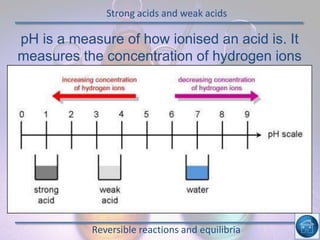
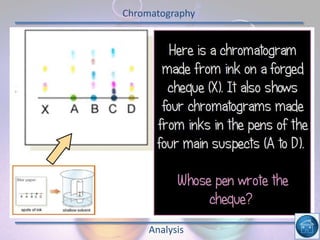
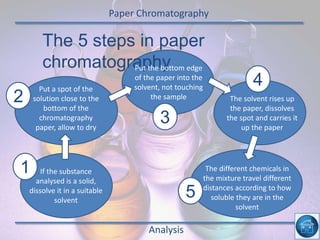
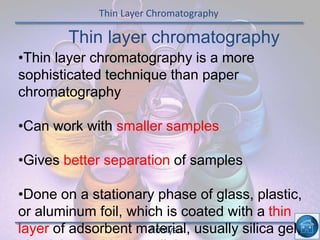
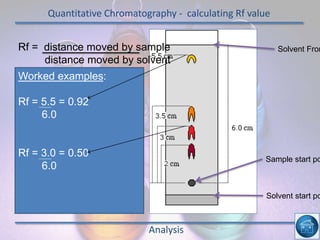
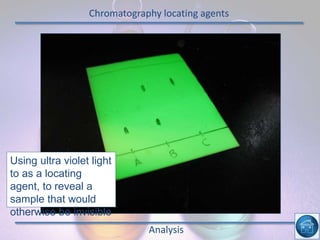
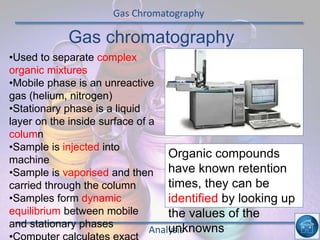
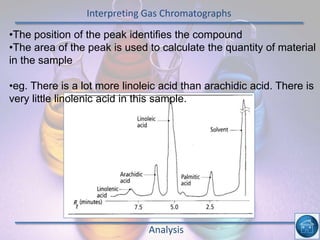
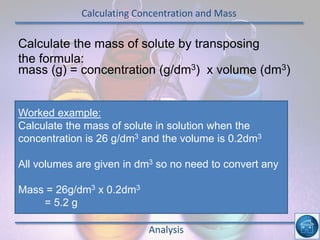
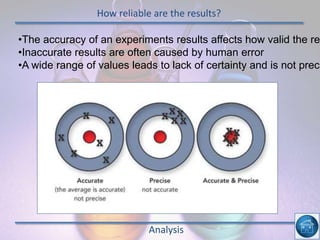
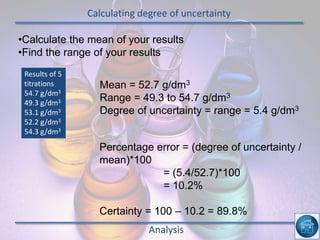
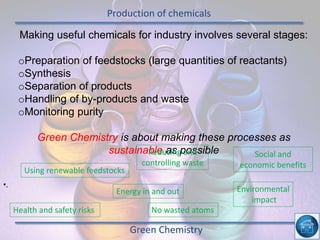
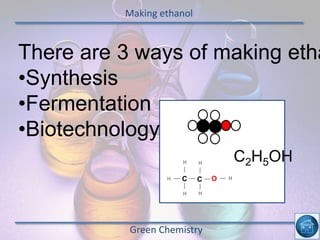
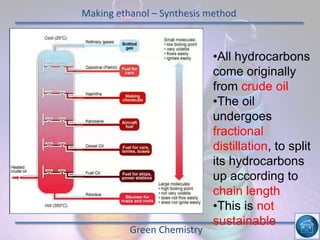
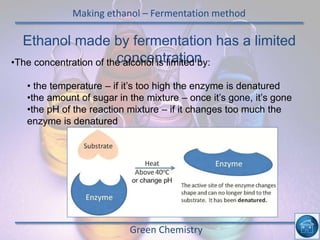
The hydrogen and oxygen reaction (example 1) gives out more heat energy than the hydrogen and chlorine reaction (example 2). In example 1, the energy change was -484 kJ, meaning 484 kJ of heat was given out. In example 2, the energy change was -185 kJ, meaning 185 kJ of heat was given out. Since -484 kJ is a larger negative number, the hydrogen and oxygen reaction releases more energy and is therefore more exothermic.