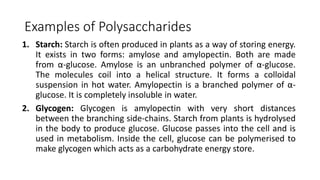
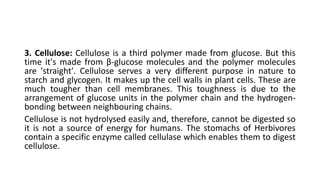
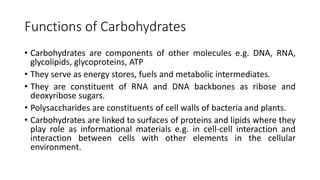
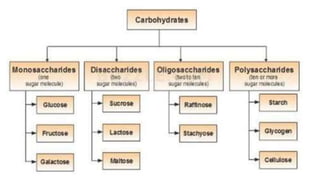
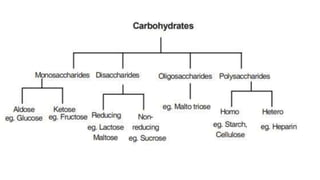
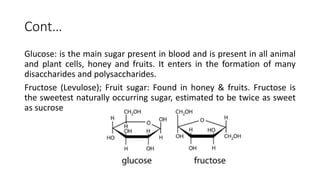
Carbohydrates are organic compounds made of carbon, hydrogen, and oxygen. They include sugars (monosaccharides and disaccharides) and starches/fibers (polysaccharides). Monosaccharides like glucose are the simplest type, while polysaccharides are long chains of monosaccharides joined by glycosidic bonds. Carbohydrates serve important functions like energy storage, structure in cell walls, and as components of other biomolecules. They are classified based on their structure as monosaccharides, disaccharides, oligosaccharides, or polysaccharides.





![Disaccharides
Two monosaccharides can join to form a disaccharide. The bond
formed between the monosaccharide is called glycosidic bond.
Disaccharides are produced from the condensation of 2
monosaccharide molecules. Examples:
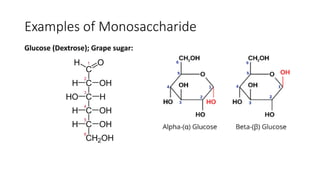
• Lactose [milk sugar]: It is composed of galactose and glucose.
• Maltose (Malt sugar): is composed of 2 molecules of glucose.
• Sucrose (Table sugar, Cane sugar, Beet sugar): is composed of glucose
and fructose
Disaccharides can be classified as reducing and non reducing sugars.](https://image.slidesharecdn.com/carbohydrates-221216085743-8dbf5b20/85/Carbohydrates-pptx-11-320.jpg)