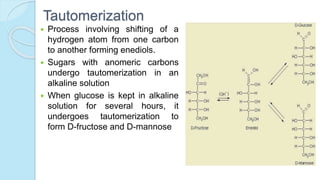
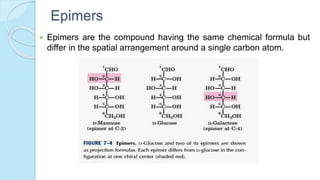
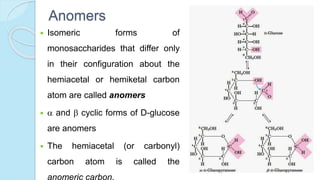
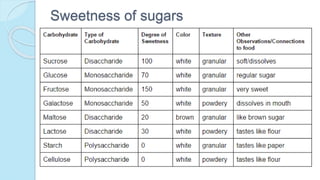
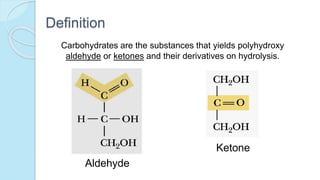
This document provides an overview of carbohydrates, including their biochemical and medical importance, classification, structure, properties, and reactions. It defines carbohydrates as substances that yield polyhydroxy aldehyde or ketones upon hydrolysis. Carbohydrates are classified as monosaccharides, oligosaccharides, or polysaccharides depending on their size. Monosaccharides can further be classified based on the number of carbons. Carbohydrates have important roles as energy sources and structural components in living organisms.




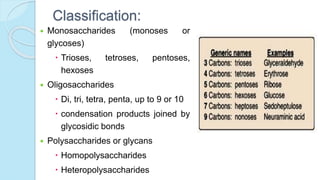
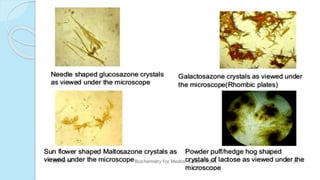
![MONOSACCHARIDES
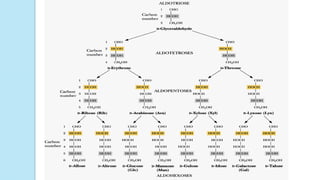
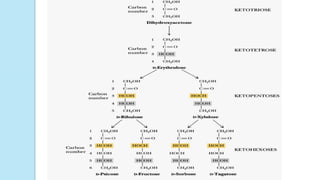
Simplest group of carbohydrate
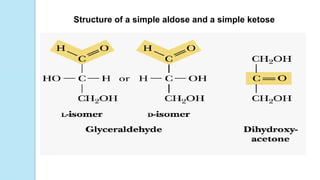
Carbohydrates that have a free carbonyl group have the suffix "-
ose." [Note: Ketoses (with some exceptions, for example, fructose)
have an additional two letters in their suffix; “-ulose” for example,
xylulose.]
Monosaccharides can be linked by glycosidic bonds to create larger
structures
Further classified based on the number of carbons and functional
group](https://image.slidesharecdn.com/carbohydrates-170911135412/85/Carbohydrates-9-320.jpg)
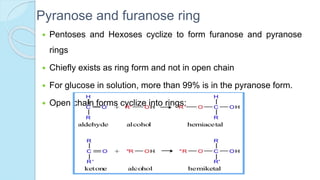
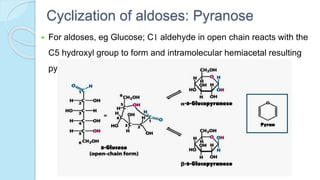
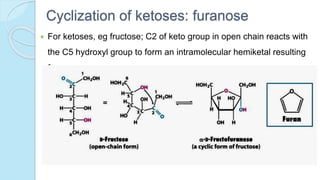
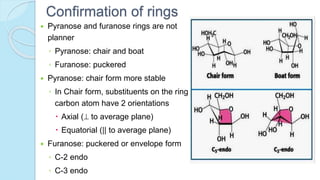


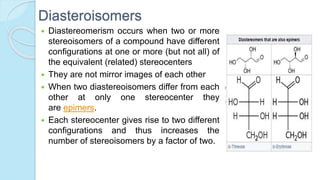

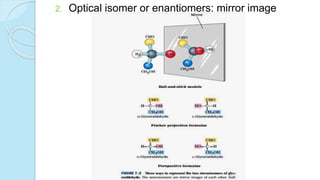


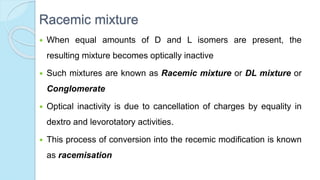

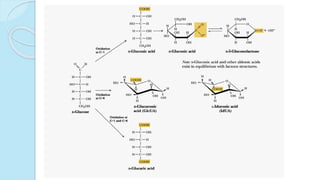
![The equilibrium mixture contains 63% beta anomer, 36% alpha
anomer of glucose and 1% open chain (glucofuranose forms)
In aqueous solution, the beta form is more predominant due to its
stable configuration
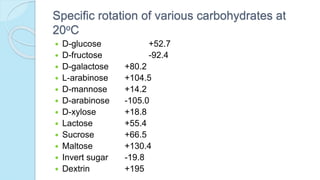
Specific optical rotation []20
D](https://image.slidesharecdn.com/carbohydrates-170911135412/85/Carbohydrates-33-320.jpg)