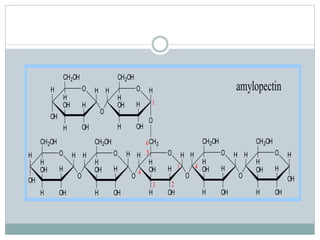
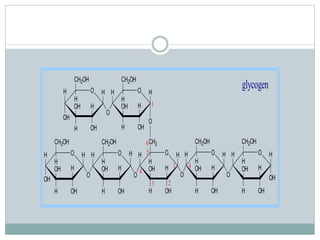
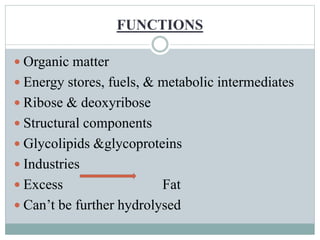
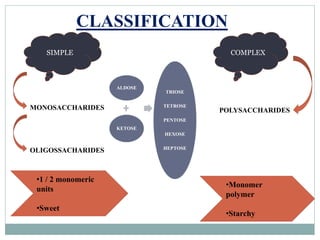
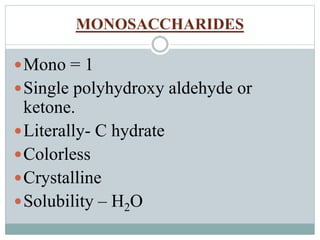
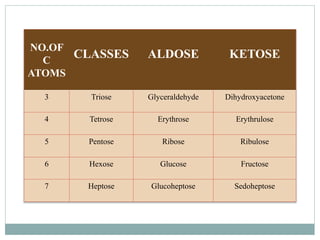
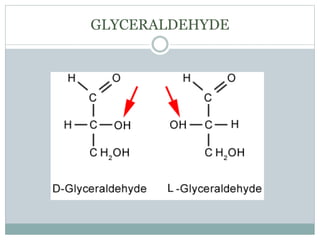
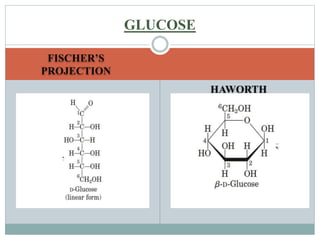
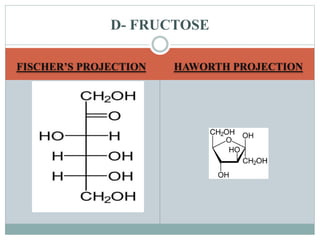
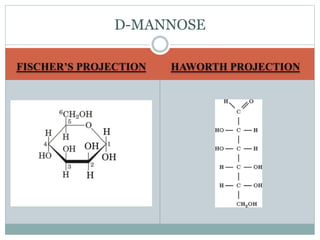
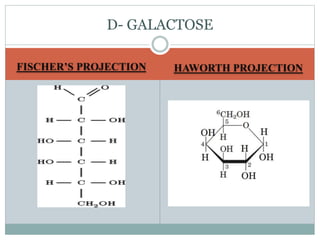
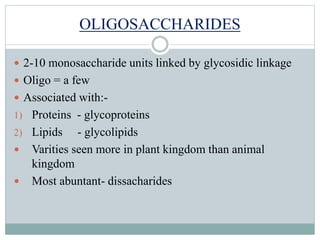
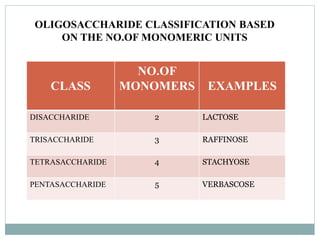
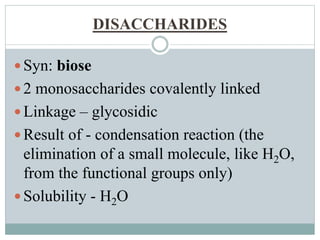
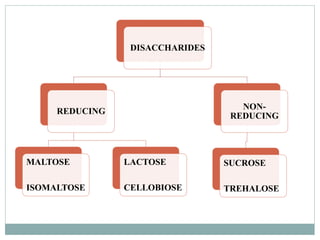
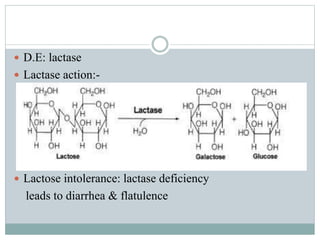
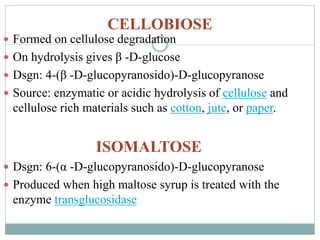
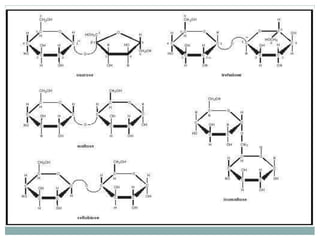
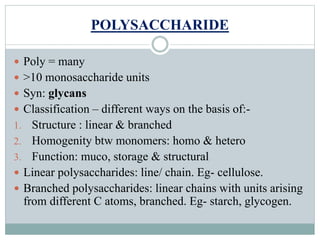
Carbohydrates are the most abundant biomolecules on Earth. They function as organic matter, energy stores, structural components, and in industries. Carbohydrates are classified as monosaccharides, oligosaccharides, or polysaccharides. Monosaccharides include aldoses and ketoses ranging from trioses to heptoses. Fischer and Haworth projections are used to represent monosaccharide structures. Oligosaccharides contain 2-10 monosaccharide units and include disaccharides like sucrose, maltose, lactose, and trehalose. Polysaccharides are polymers of monosaccharides that can be classified as storage polysaccharides like starch and glycogen or structural polysaccharides.






![FEATURE AMYLOSE AMYLOPECTIN
Content in starch 15% - 20% 80% - 85%
Branching Absent Present [α(1,6) g.l. at brancing
points]
Linkage α-1,4 α-1,4 ; α-1,6
No.of G units 200-1000 20-30
Water solubility Present Absent
Color reaction with
iodine gives
Blue Reddish-violet](https://image.slidesharecdn.com/carbohydrates-141216005129-conversion-gate02/85/Carbohydrates-35-320.jpg)