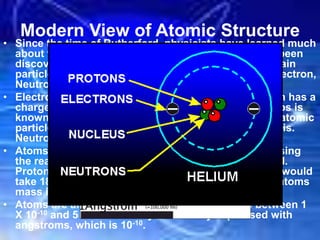
The document discusses the evolution of atomic theory and structure, starting from Democritus's idea of atoms to modern understandings of subatomic particles like electrons, protons, and neutrons. It explains key concepts such as atomic number, mass number, and isotopes, alongside significant experiments that shaped atomic theory, including Rutherford's gold foil experiment. Additionally, it introduces the periodic table's organization and the use of atomic mass units for measuring atoms.