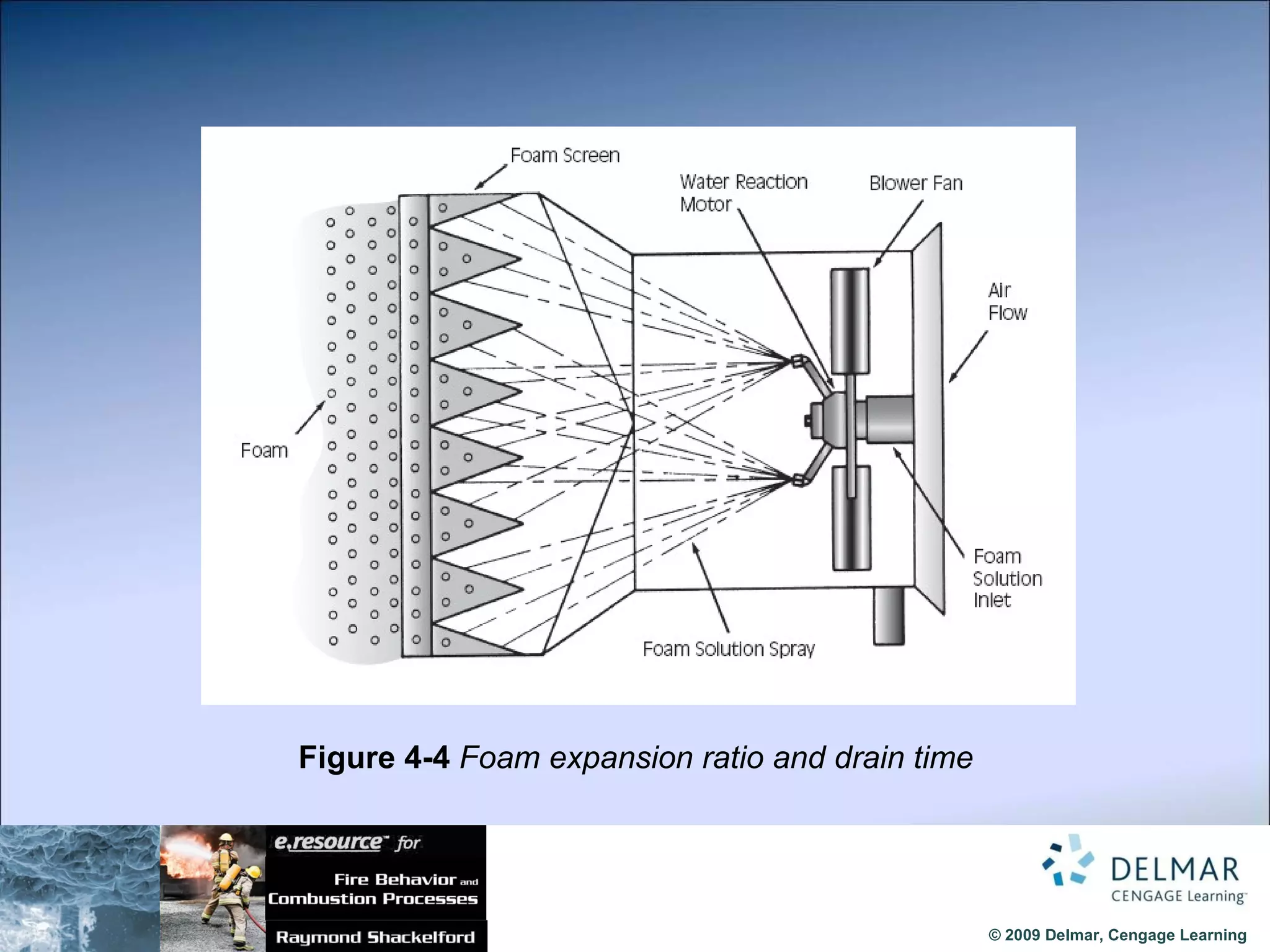
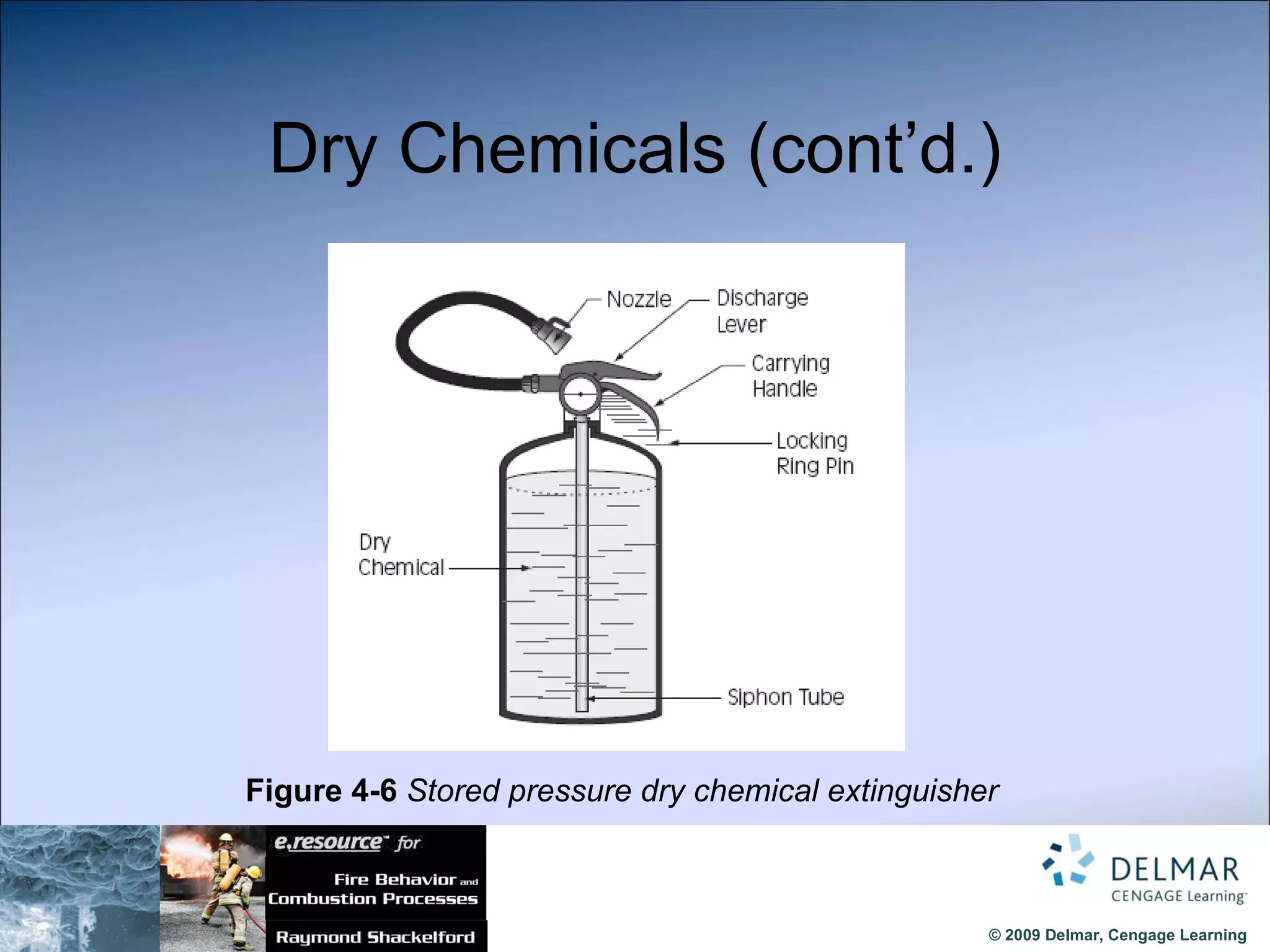
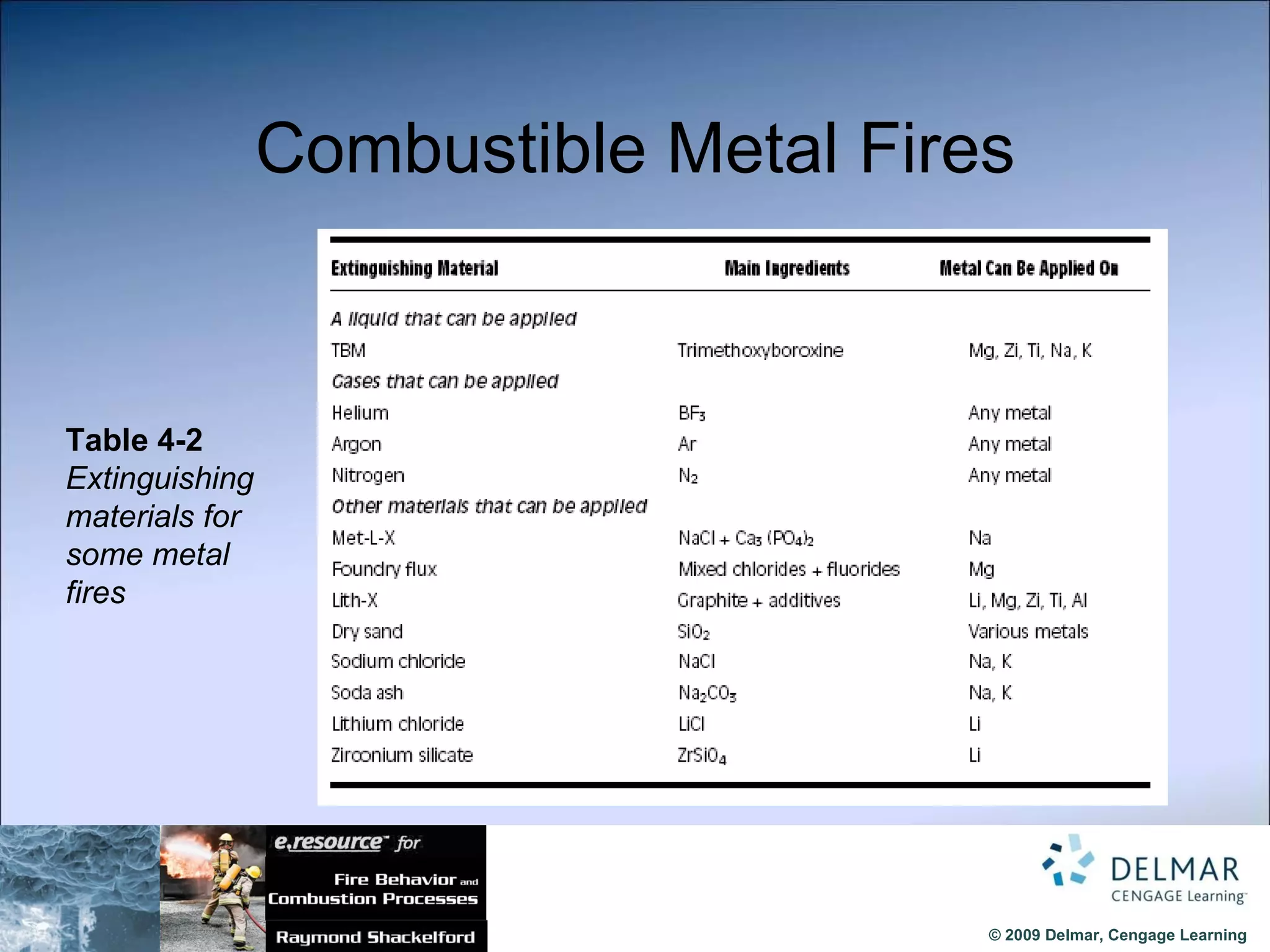
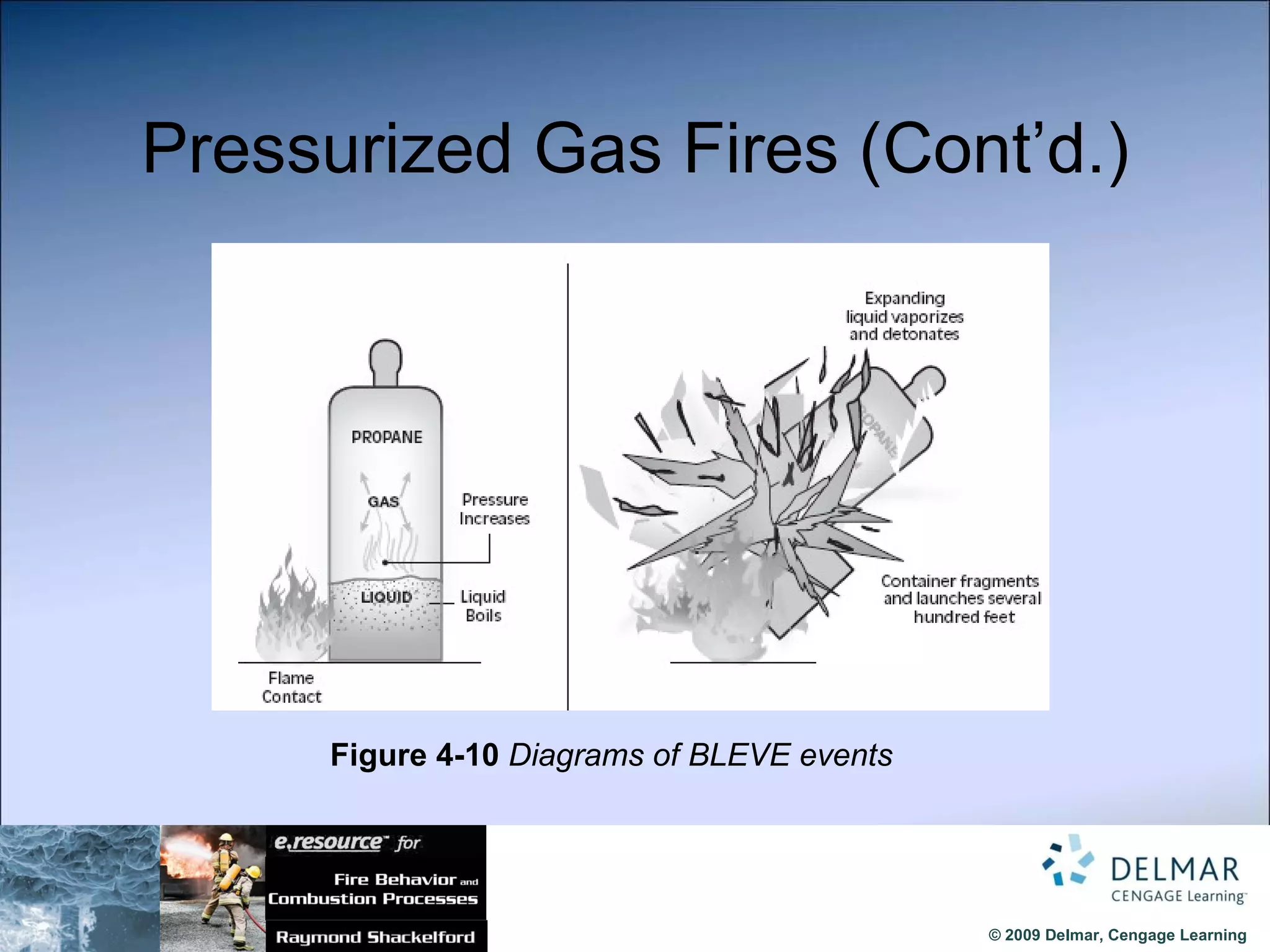
The document reviews the fire extinguishment process, detailing the five classifications of fire and the various agents used to control them, such as water, dry chemicals, and foam. It emphasizes the importance of selecting the appropriate method to effectively interrupt the combustion process, either by cooling, removing fuel, or depleting oxygen. Additionally, it discusses advancements in fire extinguishing technology, including the use of compressed air foam and ultrafine water mist systems.