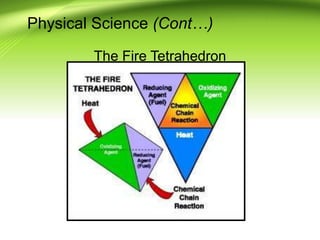
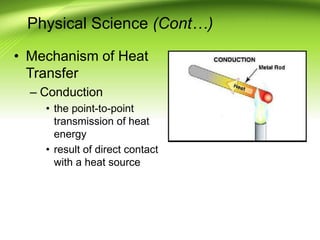
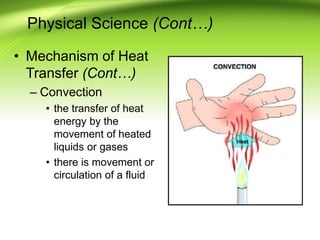
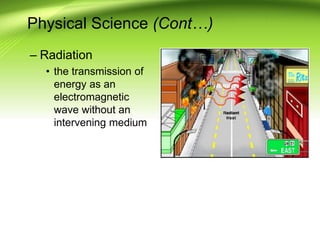
Fire requires an oxidizing agent (usually oxygen), fuel, and heat in a chemical chain reaction known as the fire tetrahedron. There are four main ways to extinguish fire: reducing temperature, removing fuel, excluding oxygen, or inhibiting the chemical reaction. Fires are classified into A, B, C, or D depending on the type of fuel. Heat transfers between objects via conduction, convection, or radiation. A fire progresses through incipient, free-burning, and smoldering phases as oxygen is consumed.