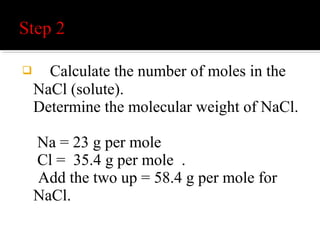
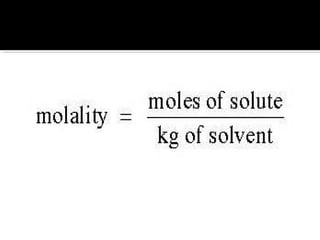
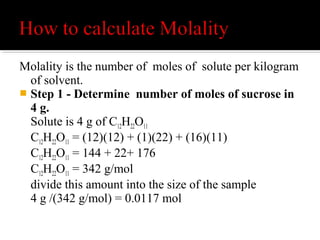
The document explains the principles of chemical reactions, including writing balanced equations, converting between moles, mass, and volume, along with examples of fermentation and production of sulfuric acid. It covers molarity and molality, detailing calculations for determining the concentration of solutions and the relevance of these concepts in chemical reactions. Multiple calculations and examples are provided to illustrate the relationships between moles, mass, and volume in chemical processes.