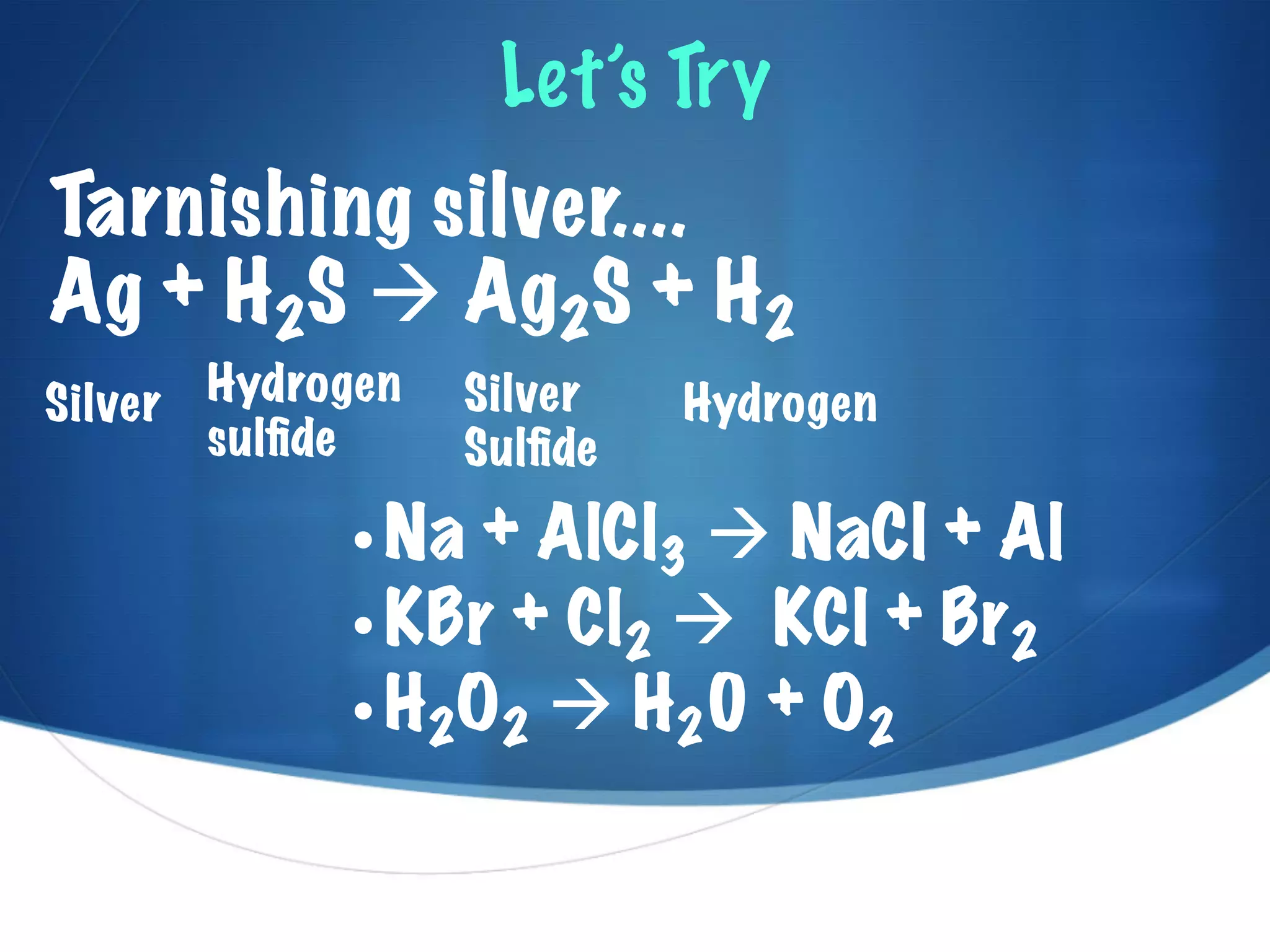
This document discusses chemical reactions and equations. It explains that chemical reactions involve substances changing partners to form new substances, while conserving mass. Chemical equations are used to represent these reactions, showing the reactants, products, and phase changes. The document also discusses how energy is often either released or absorbed during chemical reactions, depending on whether the bonds in the products are more or less stable than the reactants. Key terms like endothermic and exothermic are introduced to describe reactions that absorb or release thermal energy.