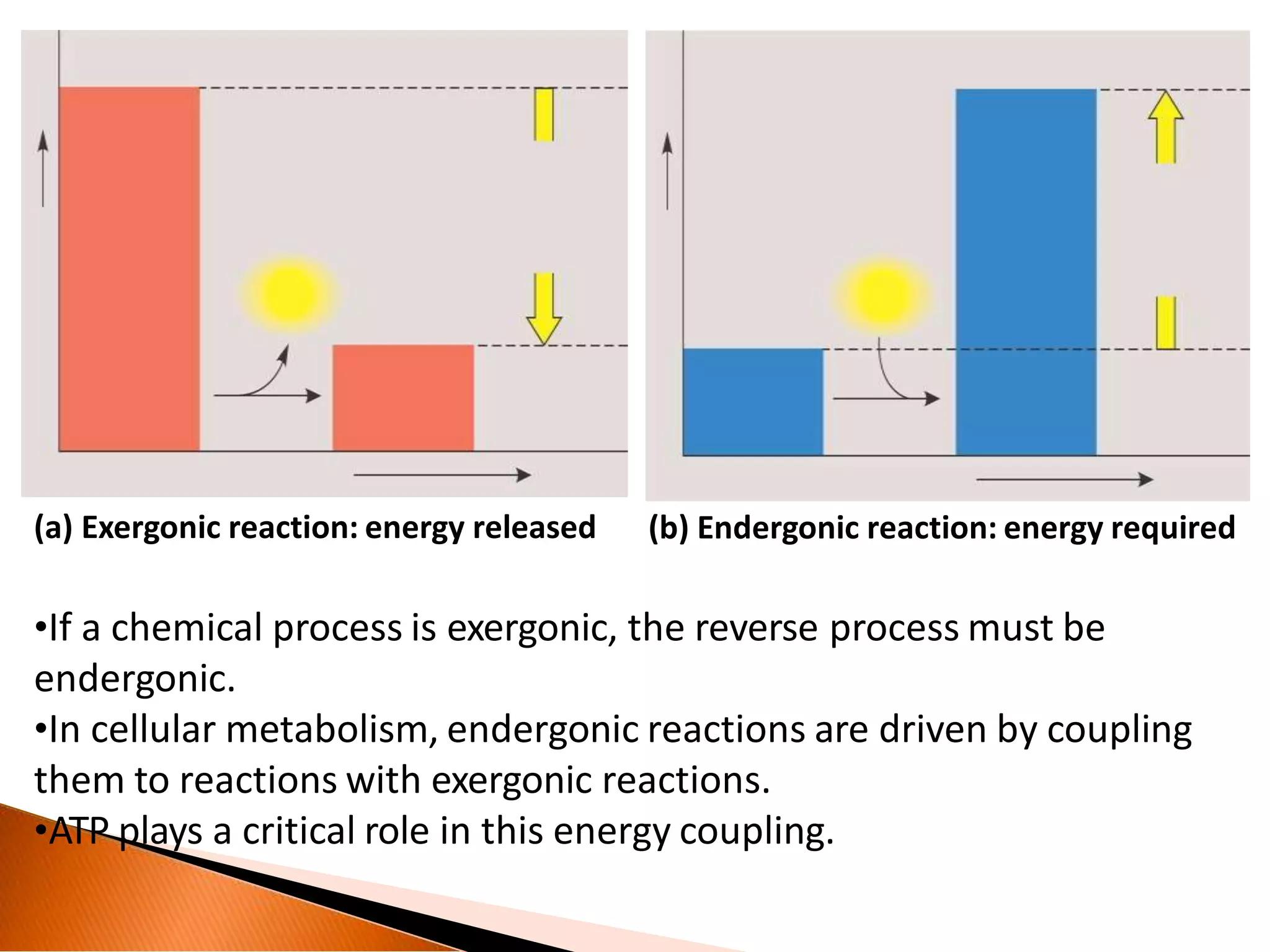
The document discusses the concept of free energy, emphasizing the significance of energy transformations in biochemical processes, including exergonic and endergonic reactions. It explains Gibbs free energy, thermodynamics laws, and the role of ATP and cyclic AMP as energy carriers in cellular metabolism. Additionally, it details the mechanisms of energy transfer, including redox potentials and the significance of high-energy bonds in biochemical reactions.






![Gibbs change in free energy (ΔG )is that portion of the total
energy change in a system available for doing work.
It is also known as the chemical potential .
ΔG= ΔH - TΔS.
Useful energy = change in Enthalpy – change in entropy
[∆G = Gibbs change in Free Energy; ∆H = Change in
Enthalpy; T = Temperature in K; ∆S = Change in Entropy]
Enthalpy, H, is the heat content of the reacting system.
It reflects the number and kinds of chemical bonds in the
reactants and products
The units of ΔG and ΔH are joules/mole or calories/mole
(recall that 1 cal equals 4.18 J);
Entropy, S, is a quantitative expression for the randomness or
disorder in a system.
units of entropy are joules/mole•degree Kelvin (J/mol•K)](https://image.slidesharecdn.com/unit1bioenergetics-211102100730/75/Bioenergetics-8-2048.jpg)