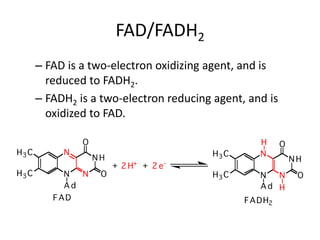
The document summarizes bioenergetics and metabolism. It discusses:
1) Metabolism, including catabolism which breaks down molecules to generate energy, and anabolism which builds molecules. The citric acid cycle and oxidative phosphorylation are described as the main catabolic pathways.
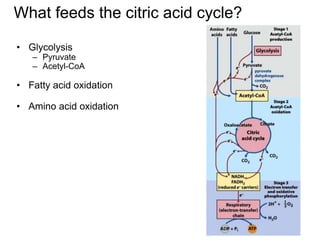
2) Glycolysis and how it feeds into the citric acid cycle, producing pyruvate. Fatty acid and amino acid oxidation also feed into the citric acid cycle.
3) The citric acid cycle which oxidizes acetyl-CoA completely to carbon dioxide, producing ATP, NADH, FADH2, and GTP. The cycle provides precursors for other processes.







































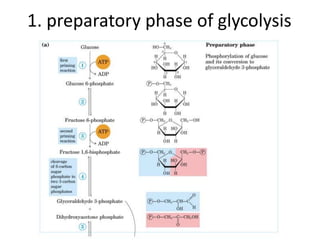



![step 2: conversion of G 6-P to F 6-P
• Phosphohexose Isomerase (aka: phosphoglucose isomerase)
– Isomerases enzymes convert between isomers
• Reversible reaction
• Direction depends on [substrate] and [product]](https://image.slidesharecdn.com/bioenergetics-160125232027/85/Bioenergetics-46-320.jpg)


















