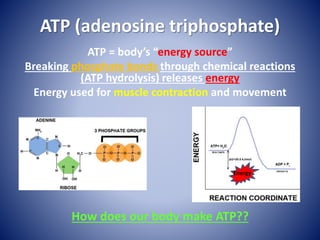
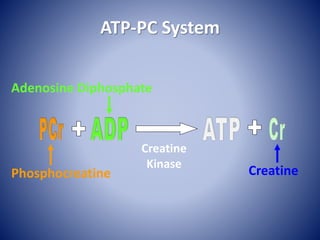
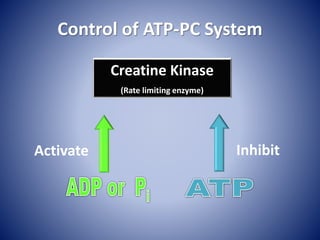
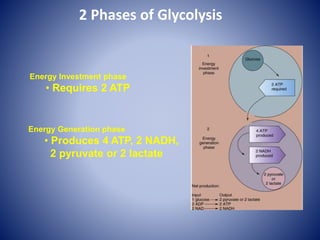
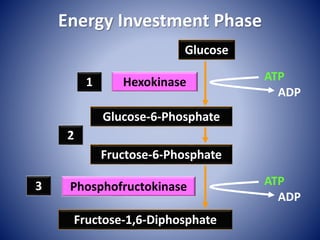
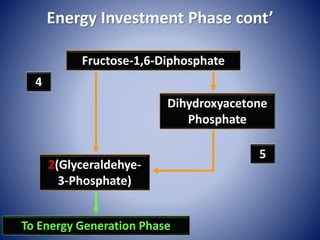
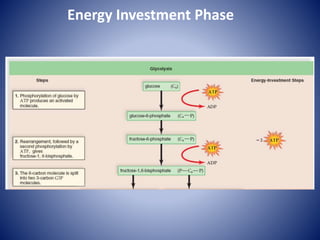
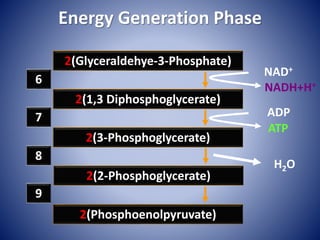
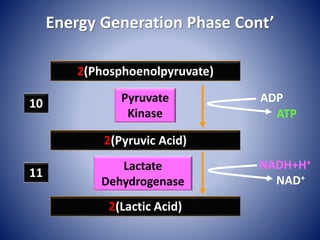
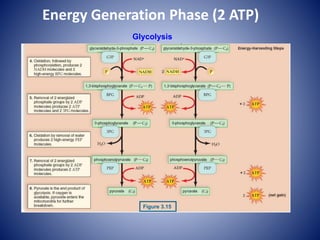
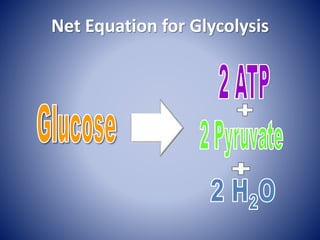
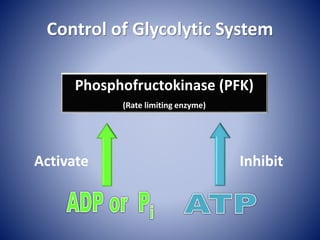
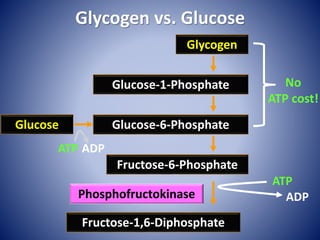
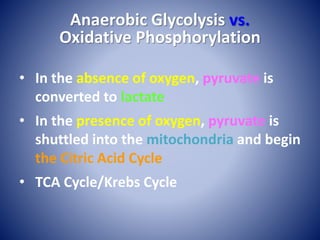
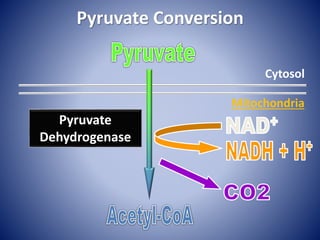
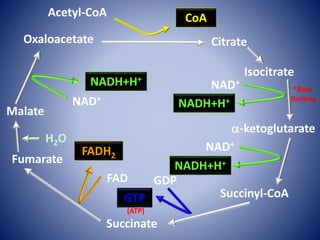
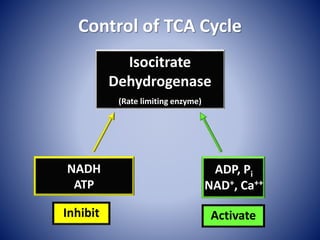
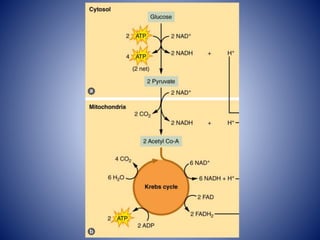
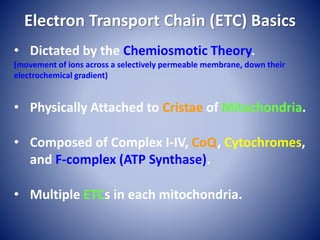
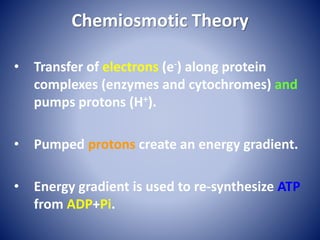
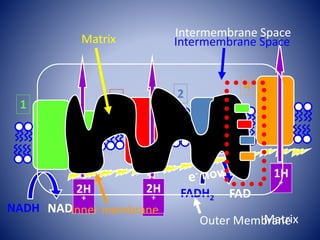
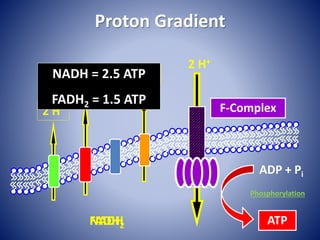
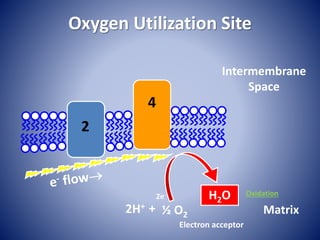
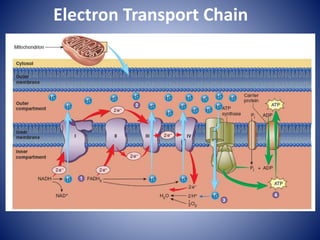
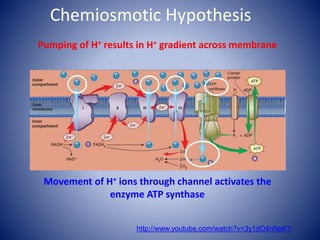
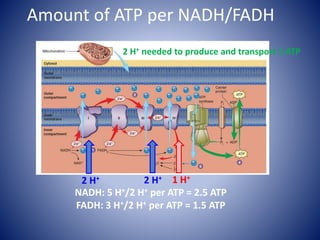
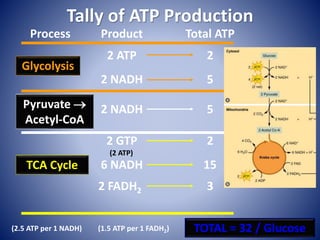
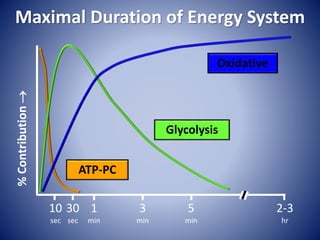
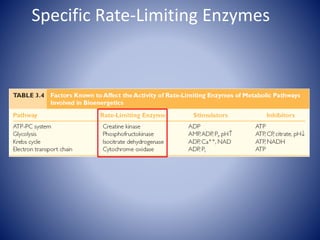
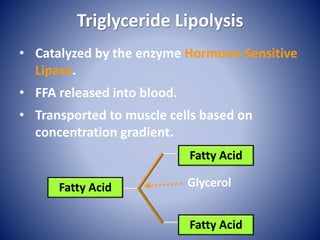
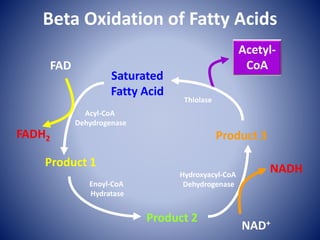
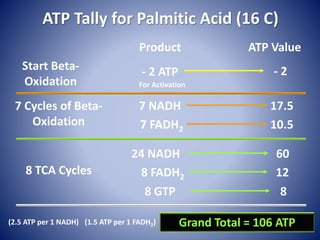
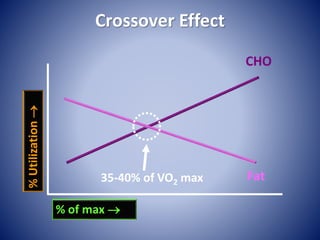
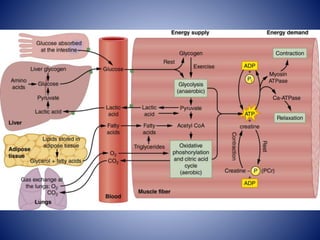
This document provides information about the three major energy systems - ATP-PCr, glycolysis, and oxidative phosphorylation - that produce energy for muscle contraction during exercise. It describes the key components, chemical reactions, and substrates involved in each system. The ATP-PCr system provides energy for up to 10 seconds of high-intensity exercise. Glycolysis can fuel exercise from 10 seconds to a few minutes by breaking down glycogen or glucose. Aerobic metabolism via the citric acid cycle and electron transport chain can sustain energy production for hours by oxidizing carbohydrates, fats, and proteins, yielding the most ATP. The document also discusses how these three systems interact to meet energy demands depending on exercise intensity and duration.