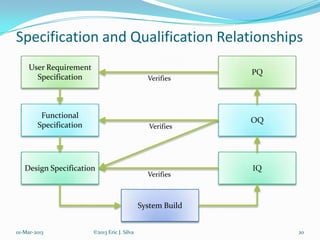
The document outlines the importance of Computer System Validation (CSV) in regulated industries, highlighting its purpose, processes, and the roles of various personnel involved. It emphasizes the necessity for compliance with FDA regulations to avoid severe repercussions such as audits and penalties. Furthermore, it discusses the responsibilities for establishing CSV policies and procedures, and details the validation process including documentation and quality management practices.









![Responsibilities
Computer system validation must be supported at different levels
within the organization through policies, standards and
documentation
Corporate/Business Unit
Site/Department Personnel
Quality Assurance and Regulatory Units
Corporate Management
IT Departments
Smaller companies can be considered a single Corporate/
Business Unit, but can also have different policies based
on Site [geographical] differences (e.g., USA, UK, India)
01-Mar-2013 ©2013 Eric J. Silva 10](https://image.slidesharecdn.com/computersystemvalidation-130313134621-phpapp01/85/Computer-System-Validation-10-320.jpg)
![Responsibilities & SOPs
Each corporate [business] unit is responsible for establishing a policy
on computer systems validation requirements.
Site or departments are responsible for:
Computer system validation Standard Operating Procedures (SOPs)
System inventory and assessment
System specific validation protocols
System specific validation documentation
SOPs must:
Comply with the Computer Systems Validation Policy and any Business
Unit policies that may apply
Be approved by the appropriate management for that site or
department
01-Mar-2013 ©2013 Eric J. Silva 11](https://image.slidesharecdn.com/computersystemvalidation-130313134621-phpapp01/85/Computer-System-Validation-11-320.jpg)

![Pre-Validation Process
Before you can validate a system, you need to identify the
systems that require validation.
Determining if a system requires validation involves
analysis of the following areas:
21 CFR Part 11 – electronic records and signatures
Manufacturing processes
Product [drug material] release or stability information
Regulatory information
Support GxP activities
All users must be trained on current SOPs related to
computer system development and validation.
01-Mar-2013 ©2013 Eric J. Silva 15](https://image.slidesharecdn.com/computersystemvalidation-130313134621-phpapp01/85/Computer-System-Validation-15-320.jpg)