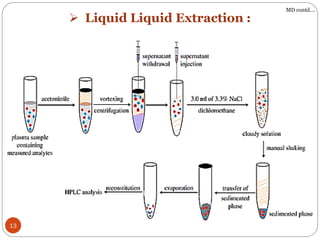
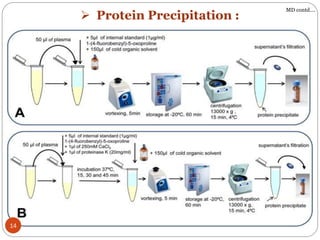
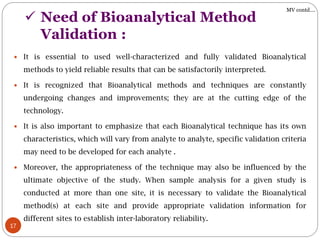
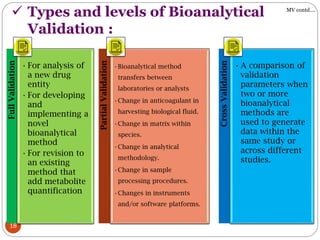
The document presents a comprehensive overview of bioanalytical methods, specifically focusing on the LC-MS/MS technique for determining drug concentrations in biological matrices. It outlines objectives, methodologies for development and validation, and essential parameters for ensuring reliability and consistency in bioanalytical results. Additionally, it emphasizes the critical need for method validation to ensure reproducibility and accuracy across different laboratories.