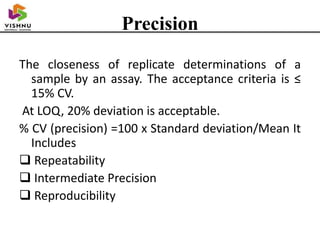
This document discusses bioanalytical method validation parameters outlined in the 2018 FDA guidance. It defines a bioanalytical method as measuring drug or metabolite concentrations in biological matrices like plasma or urine. Validation ensures a method reproducibly provides reliable results and includes specificity, accuracy, precision, recovery, matrix effects, stability, calibration curves and quality control samples. The key validation parameters are selectivity, sensitivity, accuracy, precision, recovery, matrix effects and stability which are evaluated during method development and validation.