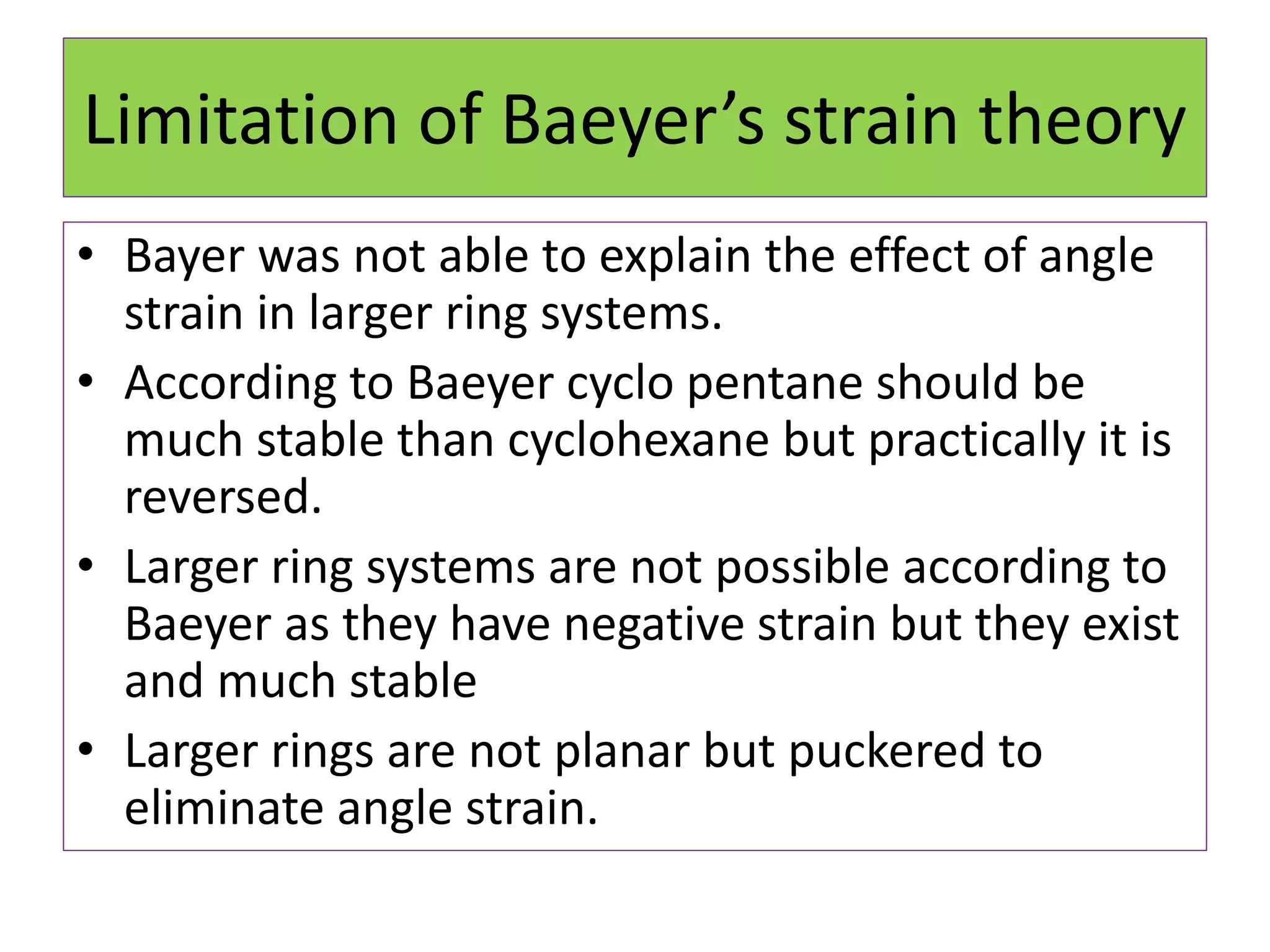
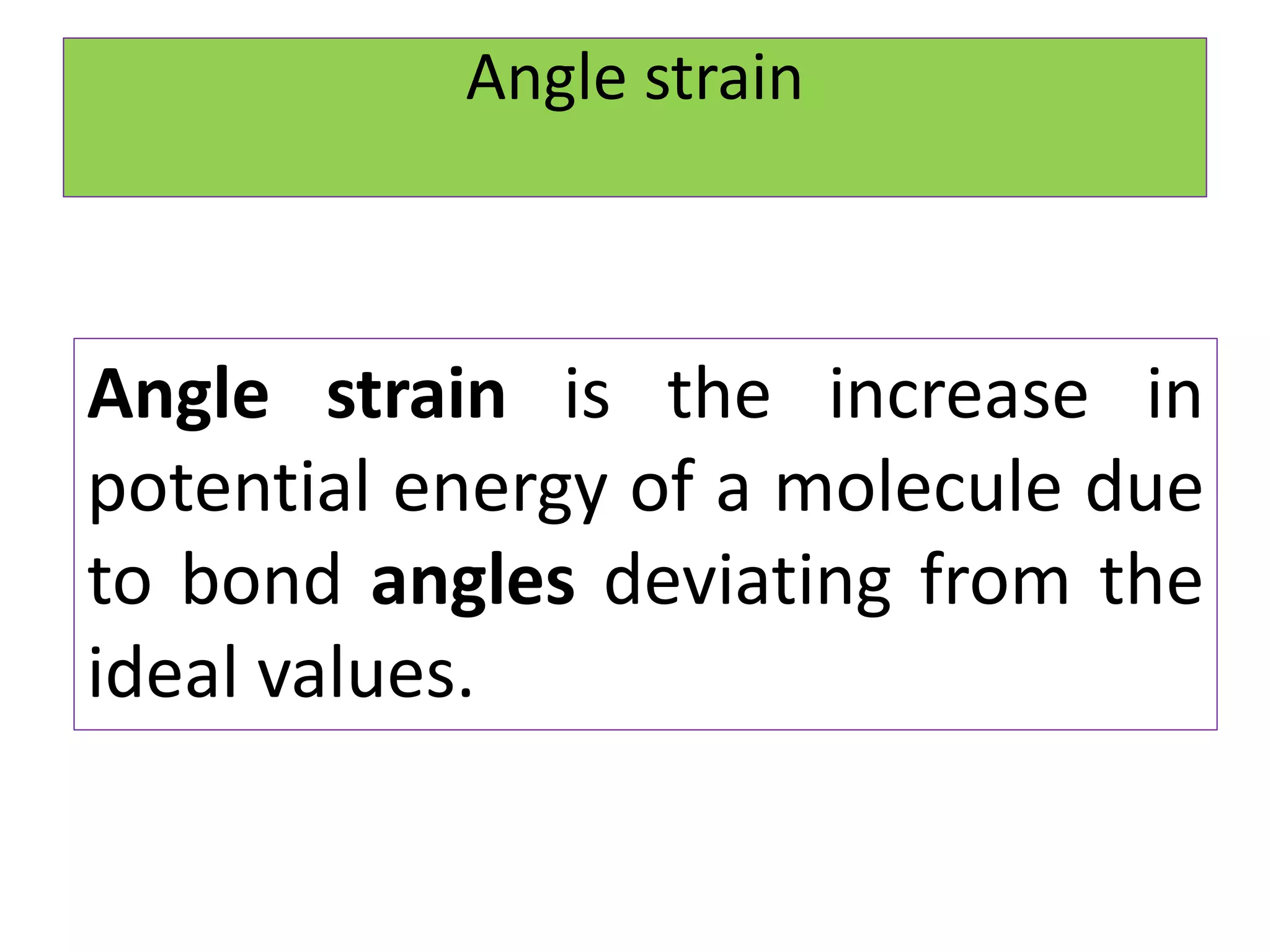
The document discusses Adolf von Baeyer's 1885 strain theory to explain the relative stability of cycloalkanes. Baeyer proposed that any deviation of bond angles from the ideal tetrahedral value of 109°28' results in angle strain. Cyclopropane has the highest strain due to 60° bond angles. Cyclopentane has the lowest strain, closest to ideal angles. While the theory explains smaller rings, it fails for cyclohexane and above, which are stable despite predicted strain. Limitations include inability to explain stability of larger rings and non-planar conformations that reduce strain.


























![Baeyer proposed [incorrectly] there should
be a certain amount of strain in
cyclohexane , further he suggested
[incorrectly]that as one proceed to
cycloheptane, cyclooctane, the deviation of
the bond angle is progressively larger and
the molecules would become progressively
more strained.](https://image.slidesharecdn.com/bayersstraintheory-201203044039/75/Bayers-strain-theory-28-2048.jpg)