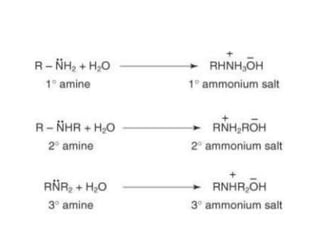
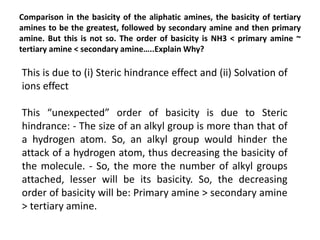
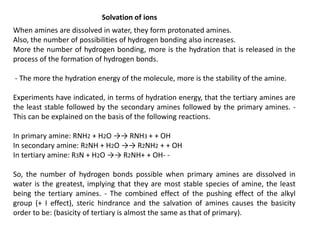
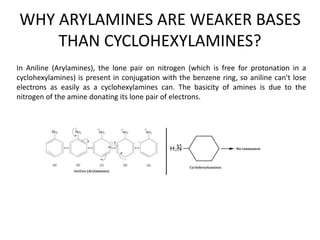
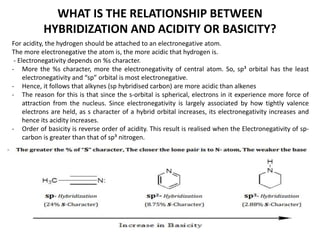
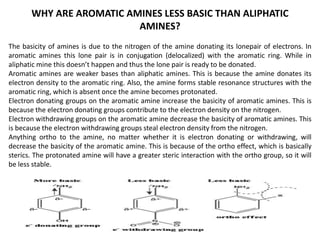
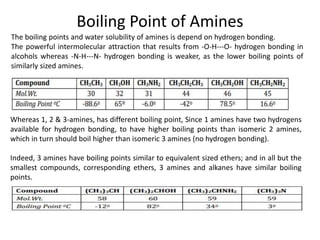
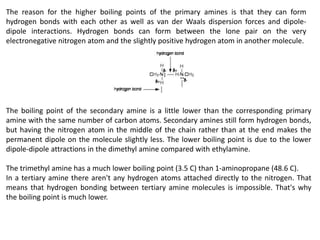
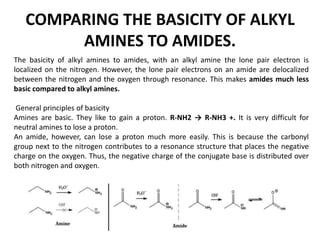
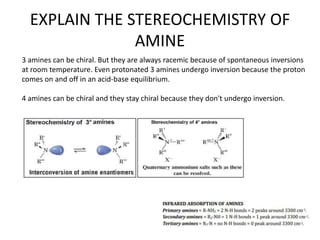
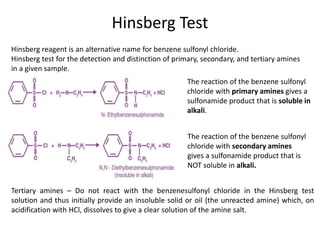
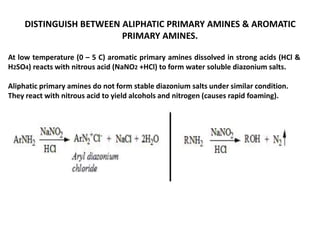
Here are the key differences between aliphatic primary amines and aromatic primary amines:
- Reactivity with nitrous acid (NaNO2 + HCl):
Aromatic primary amines form water-soluble diazonium salts at low temperatures (0-5°C).
Aliphatic primary amines react to yield alcohols and nitrogen gas, causing rapid foaming. They do not form stable diazonium salts.
- Structure:
Aromatic primary amines have the amino group attached directly to an aromatic ring.
Aliphatic primary amines have the amino group attached to an open chain alkyl group.
- Hybridization of nitrogen: