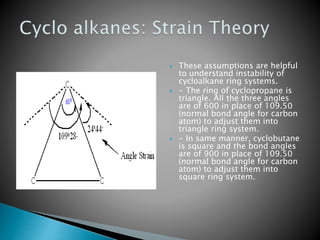
The document discusses cyclohexane conformations and Baeyer's strain theory. It explains that the chair conformation is the most stable configuration for cyclohexane, while the boat conformation is less stable due to steric interactions. Baeyer's strain theory postulates that deviations from ideal tetrahedral bond angles impose strain on a molecule, with greater deviations resulting in greater instability. The theory explains the reactivity of small ring cycloalkanes like cyclopropane.