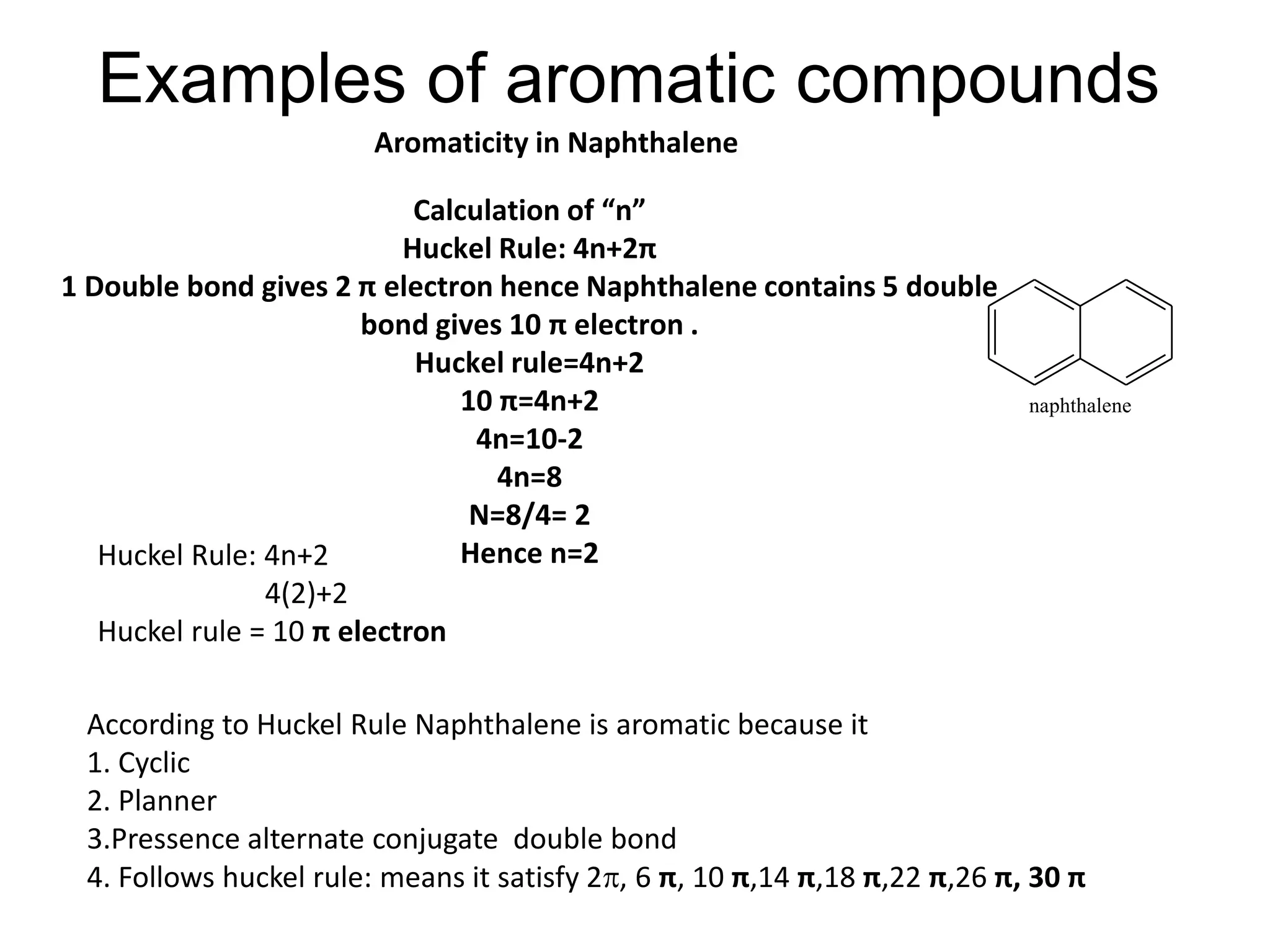
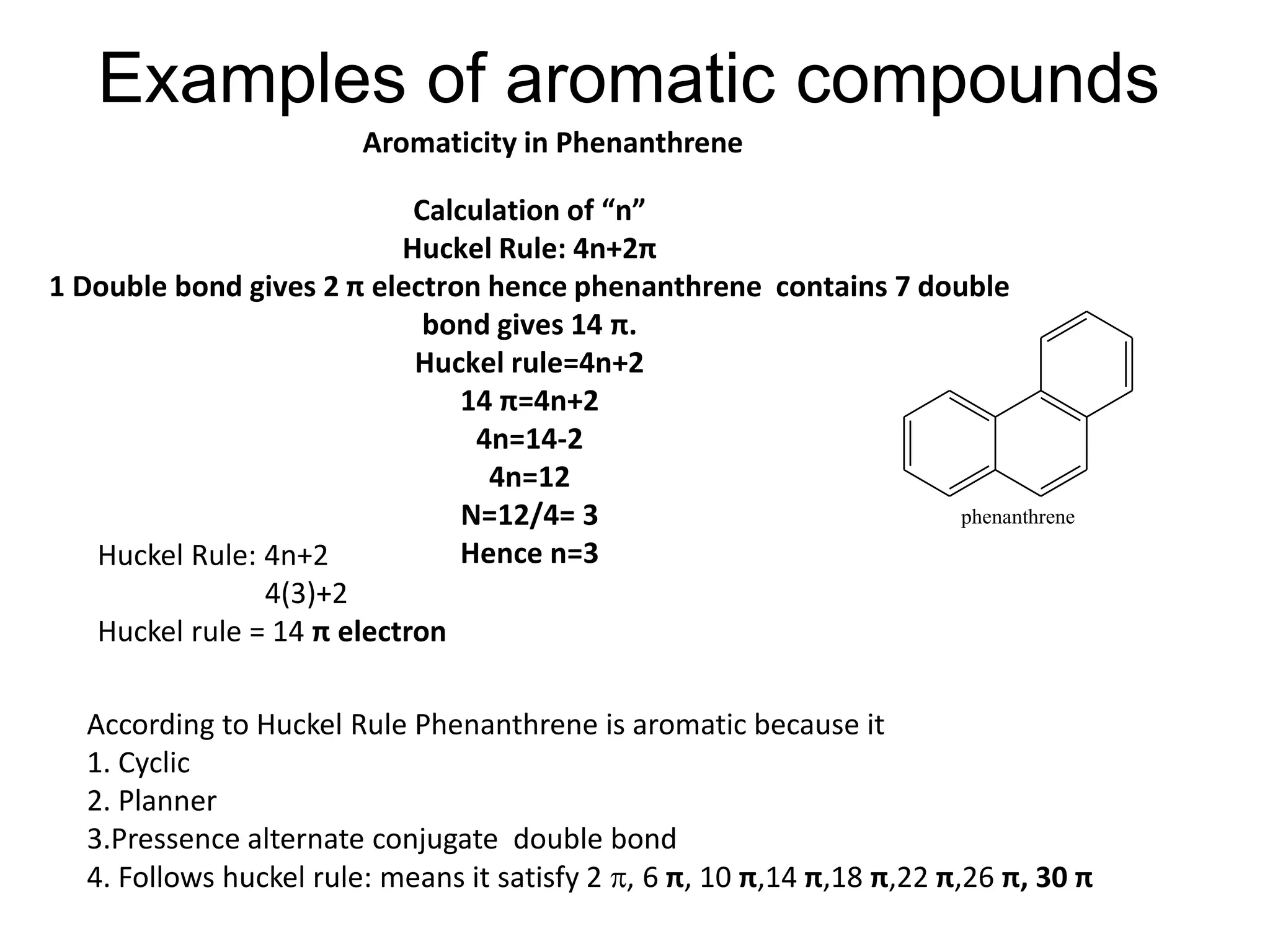
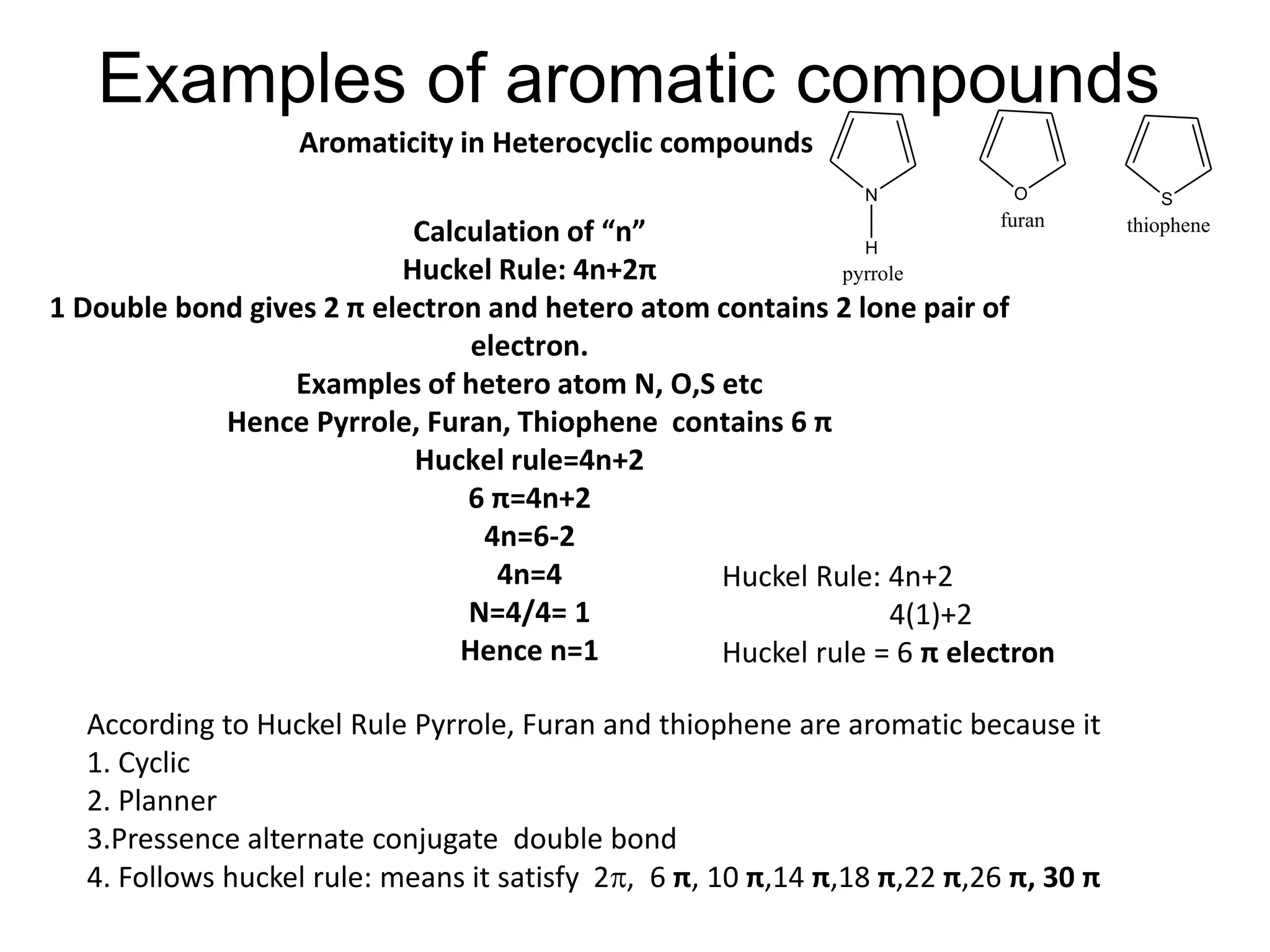
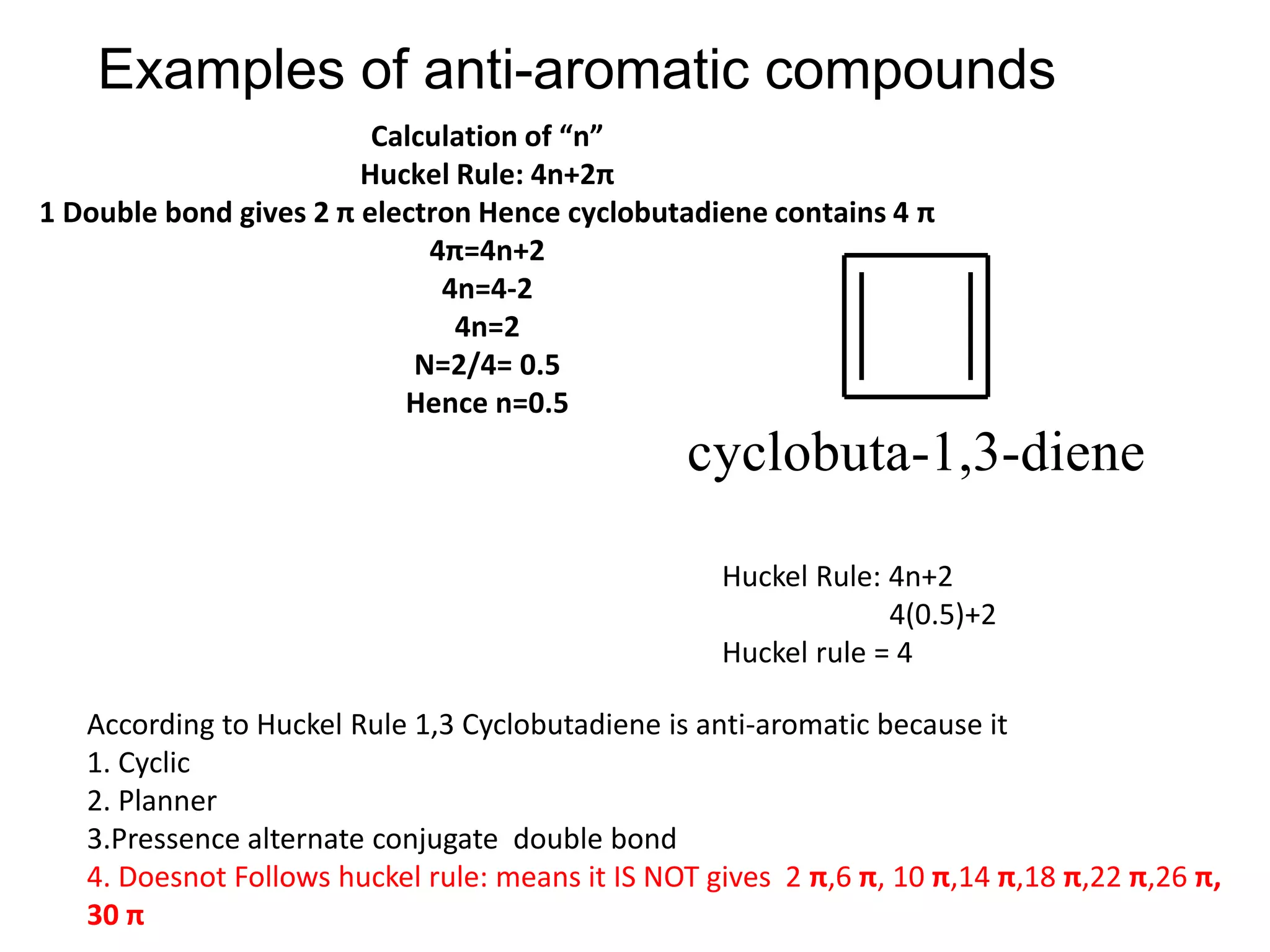
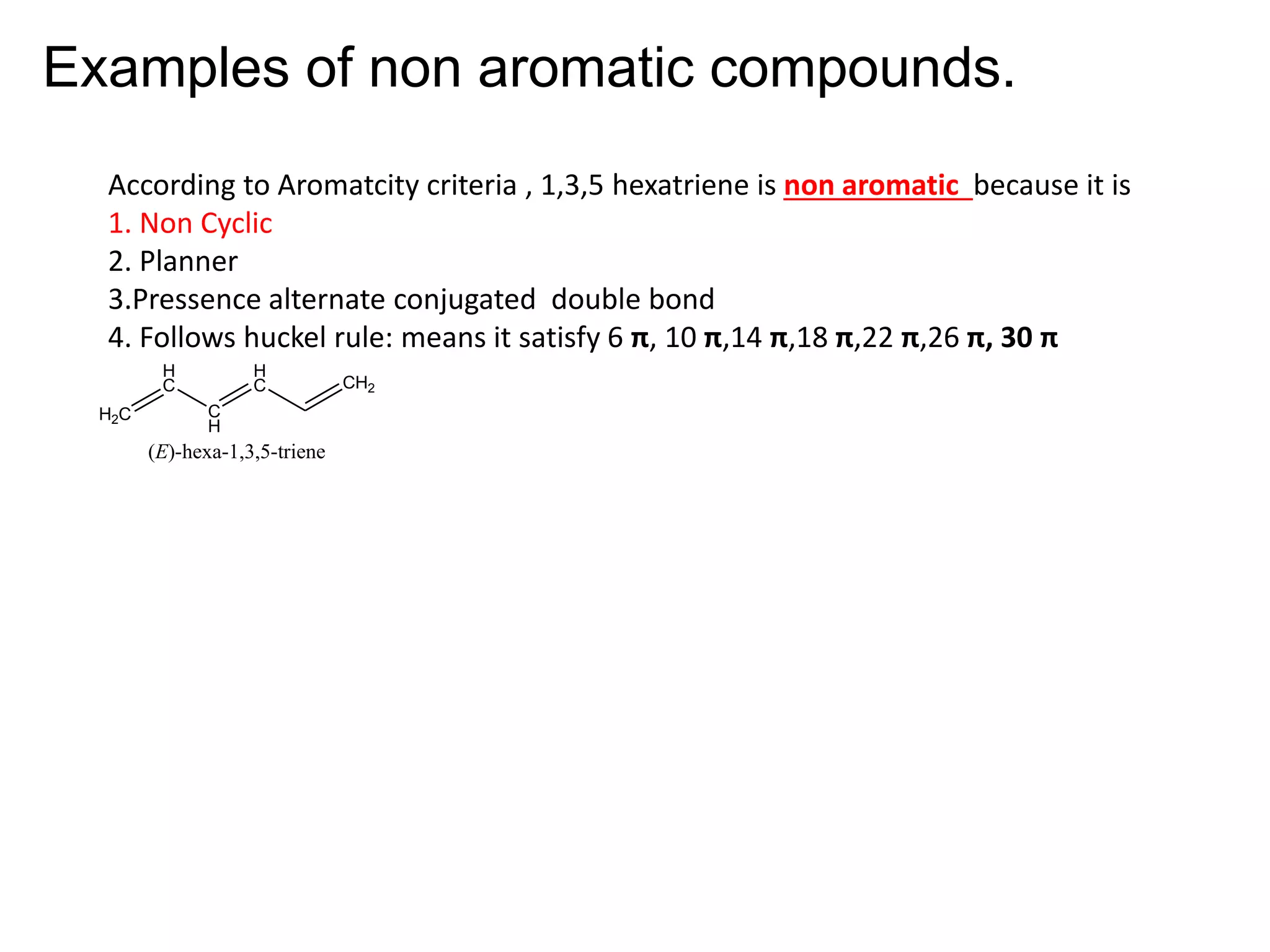
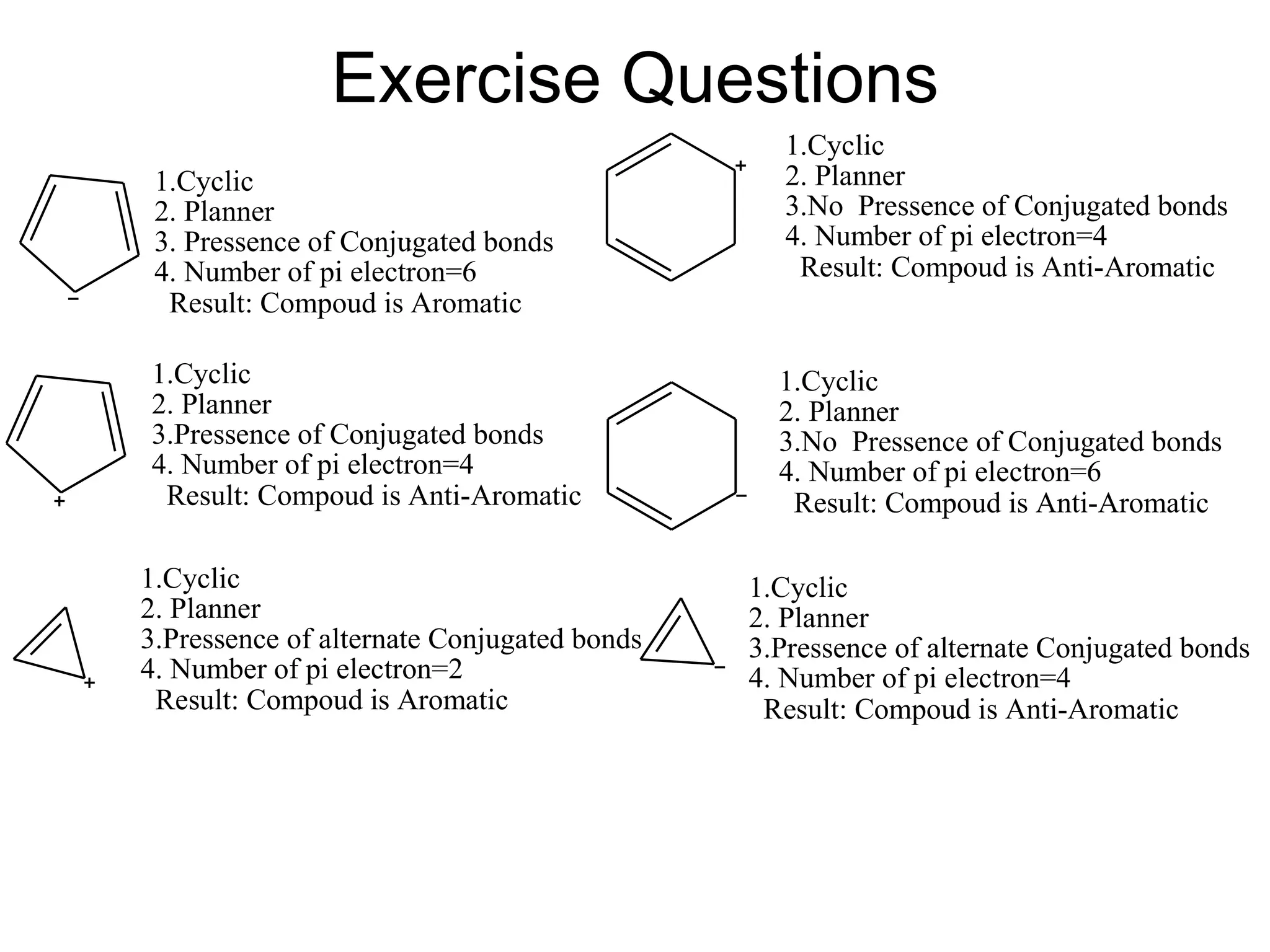
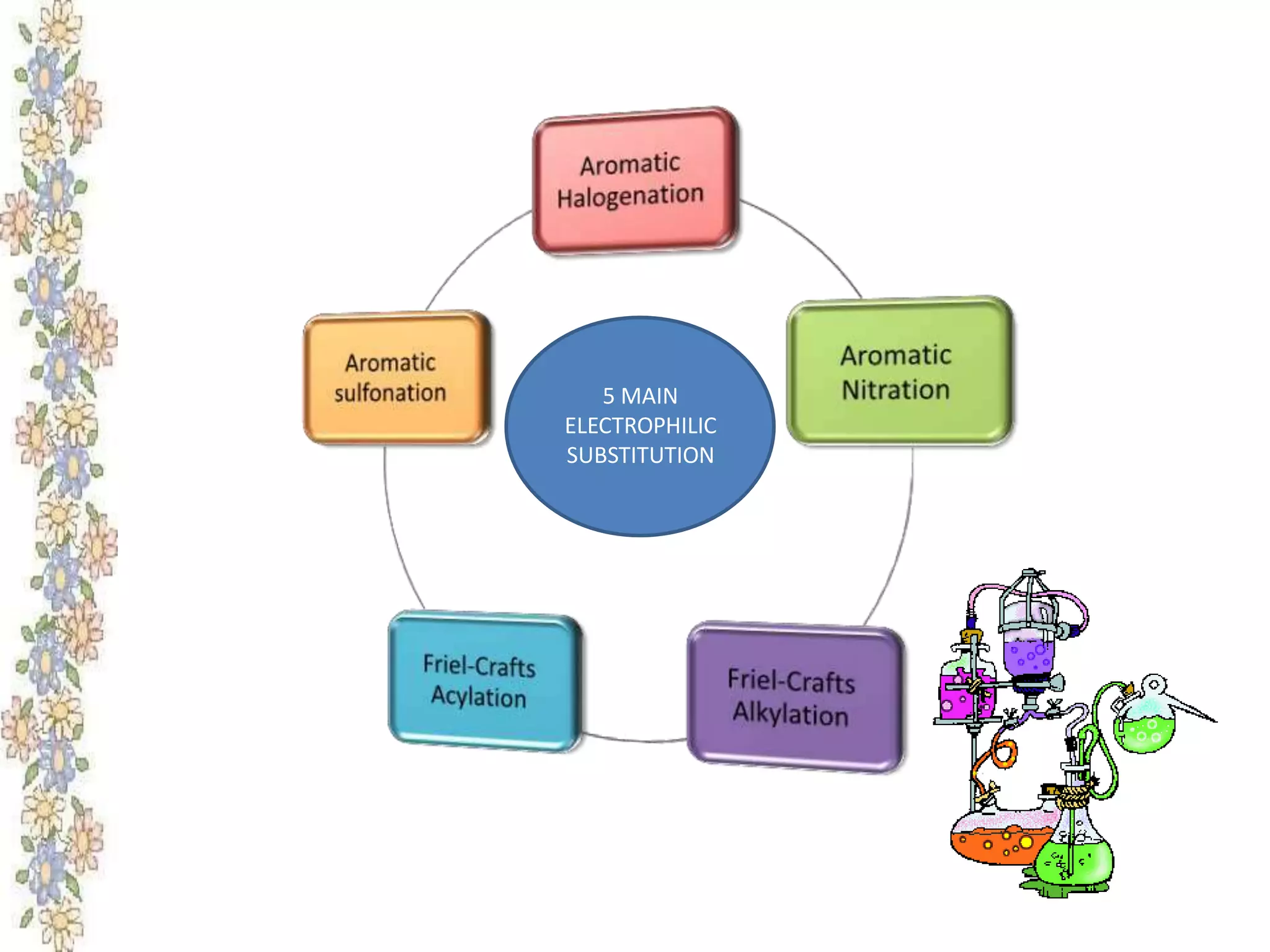
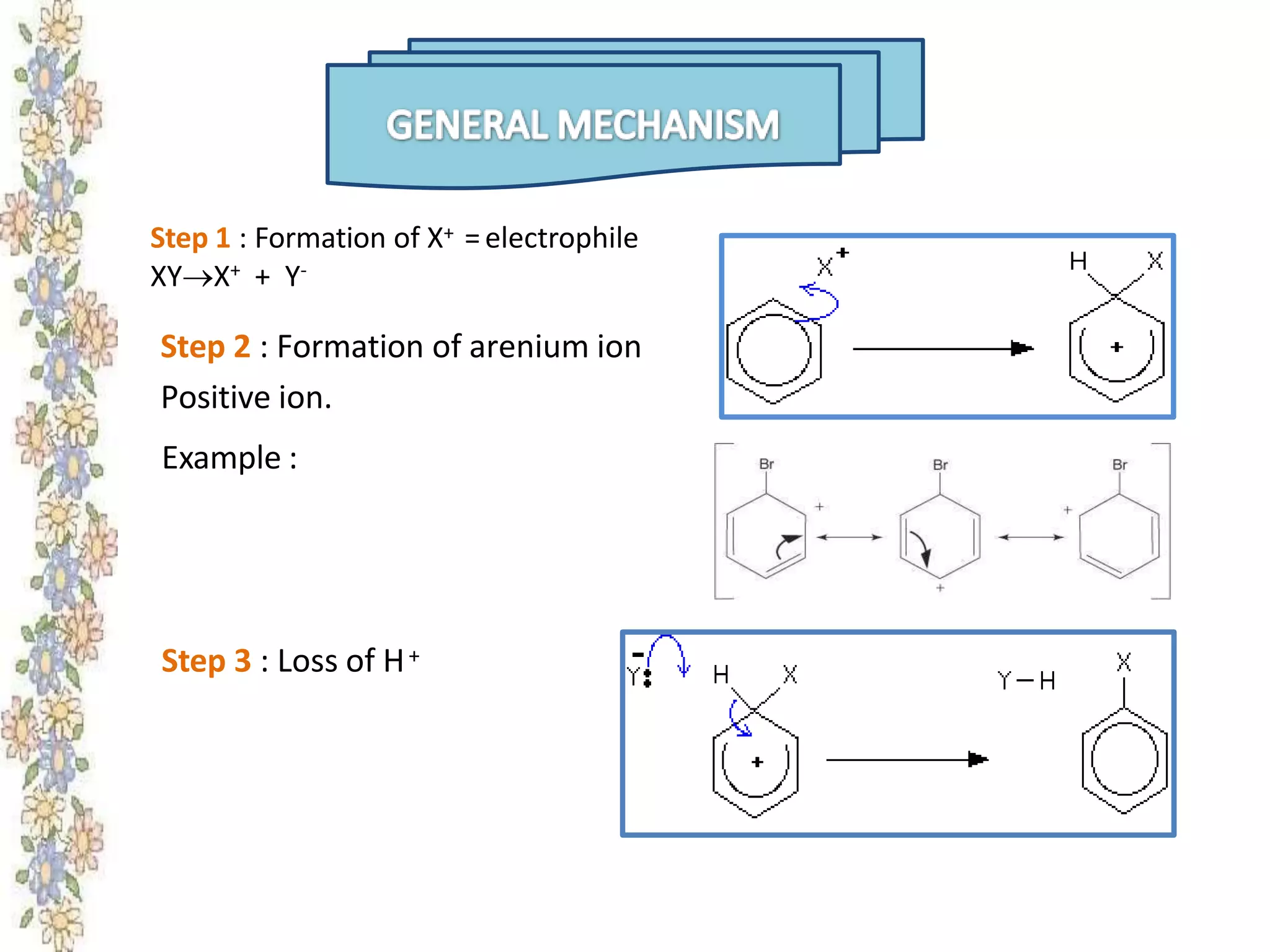
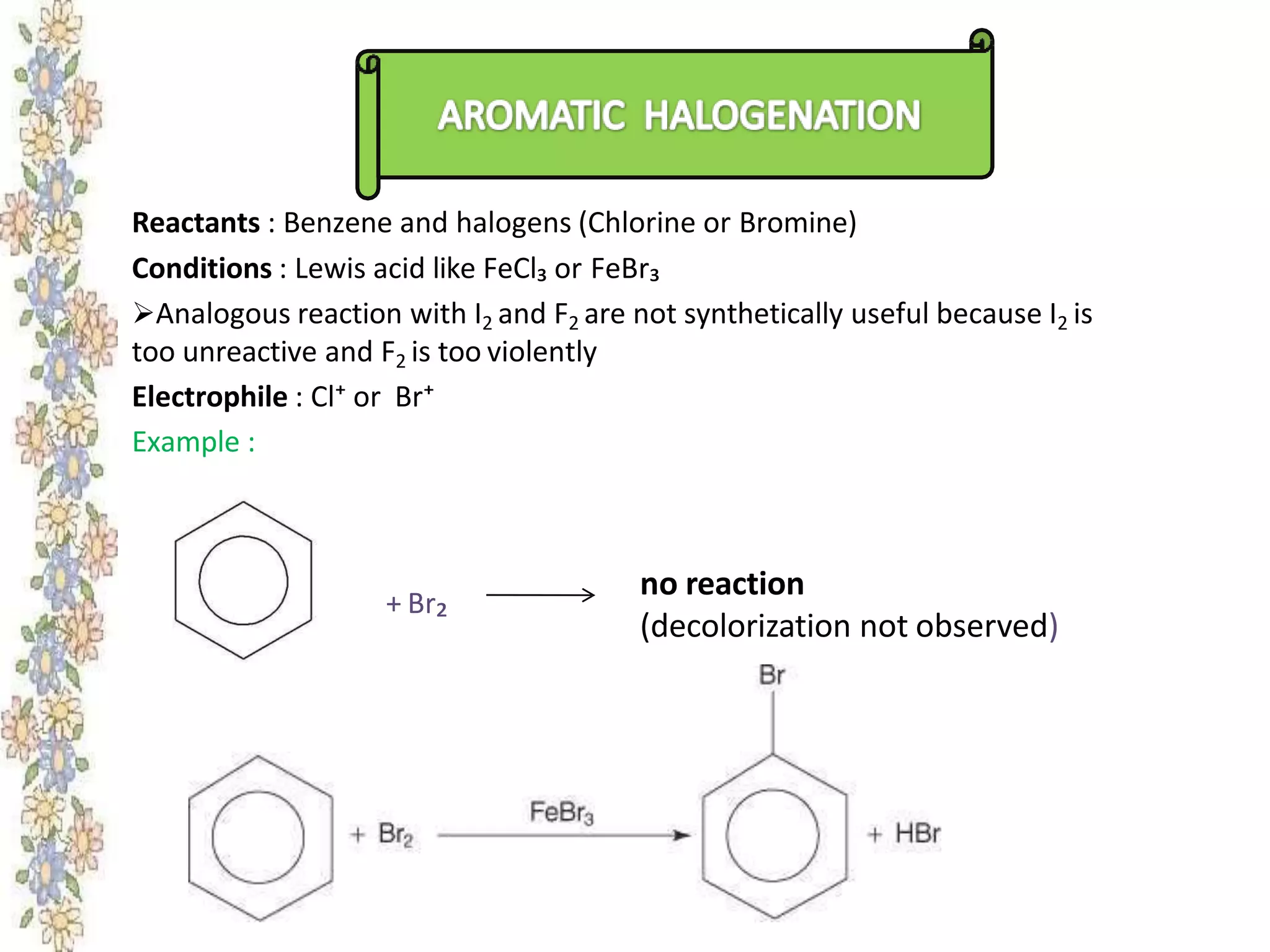
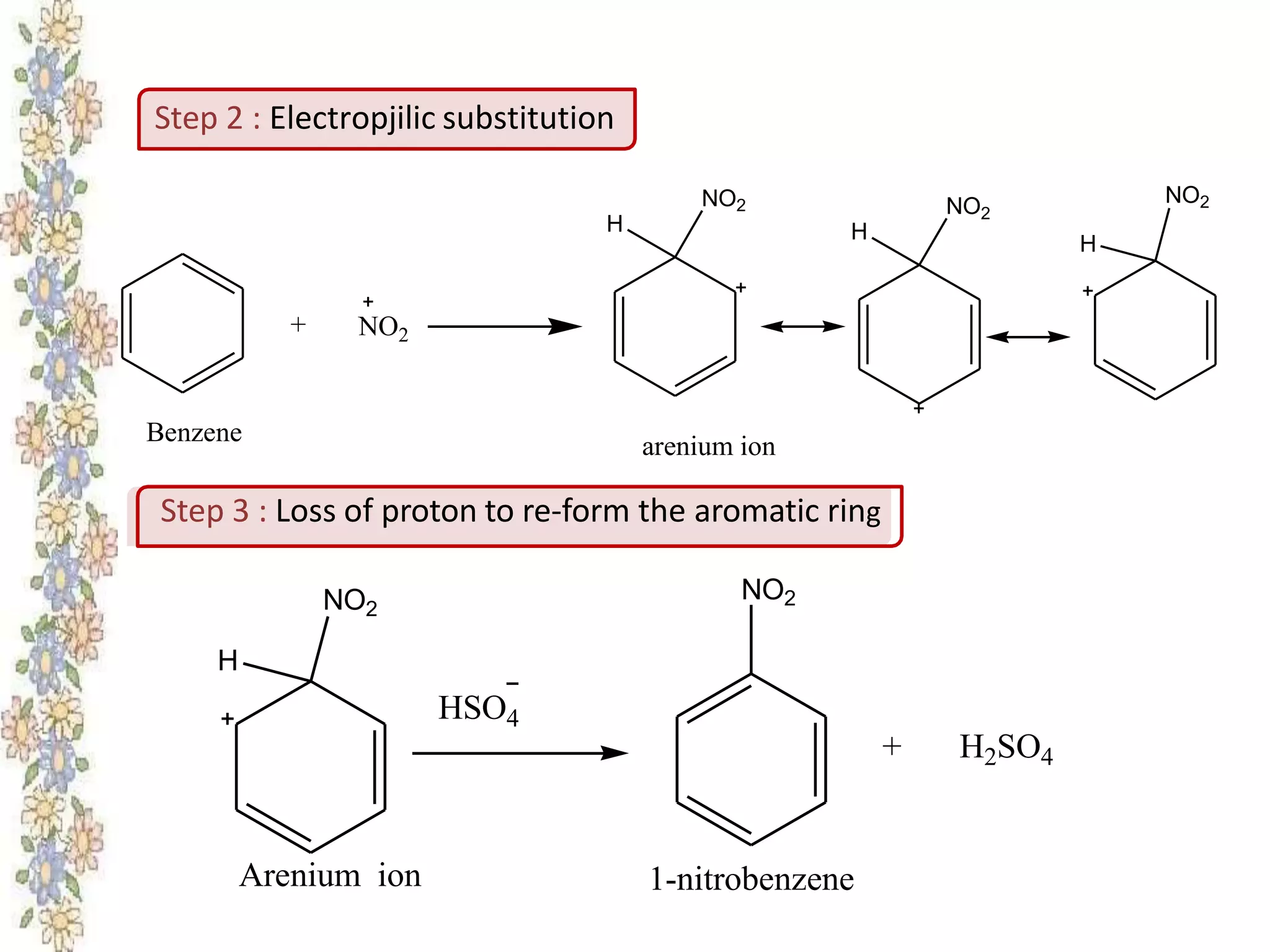
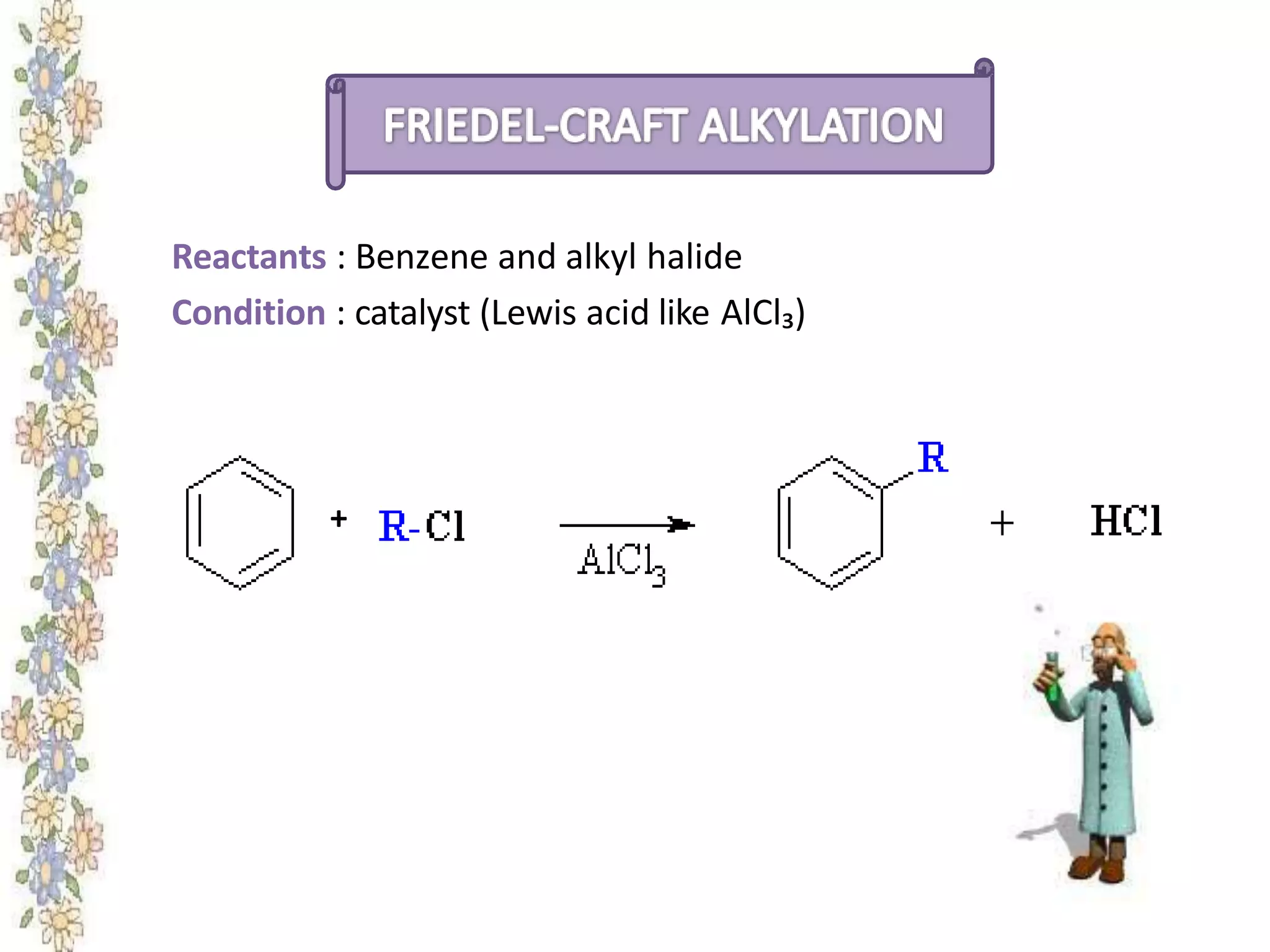
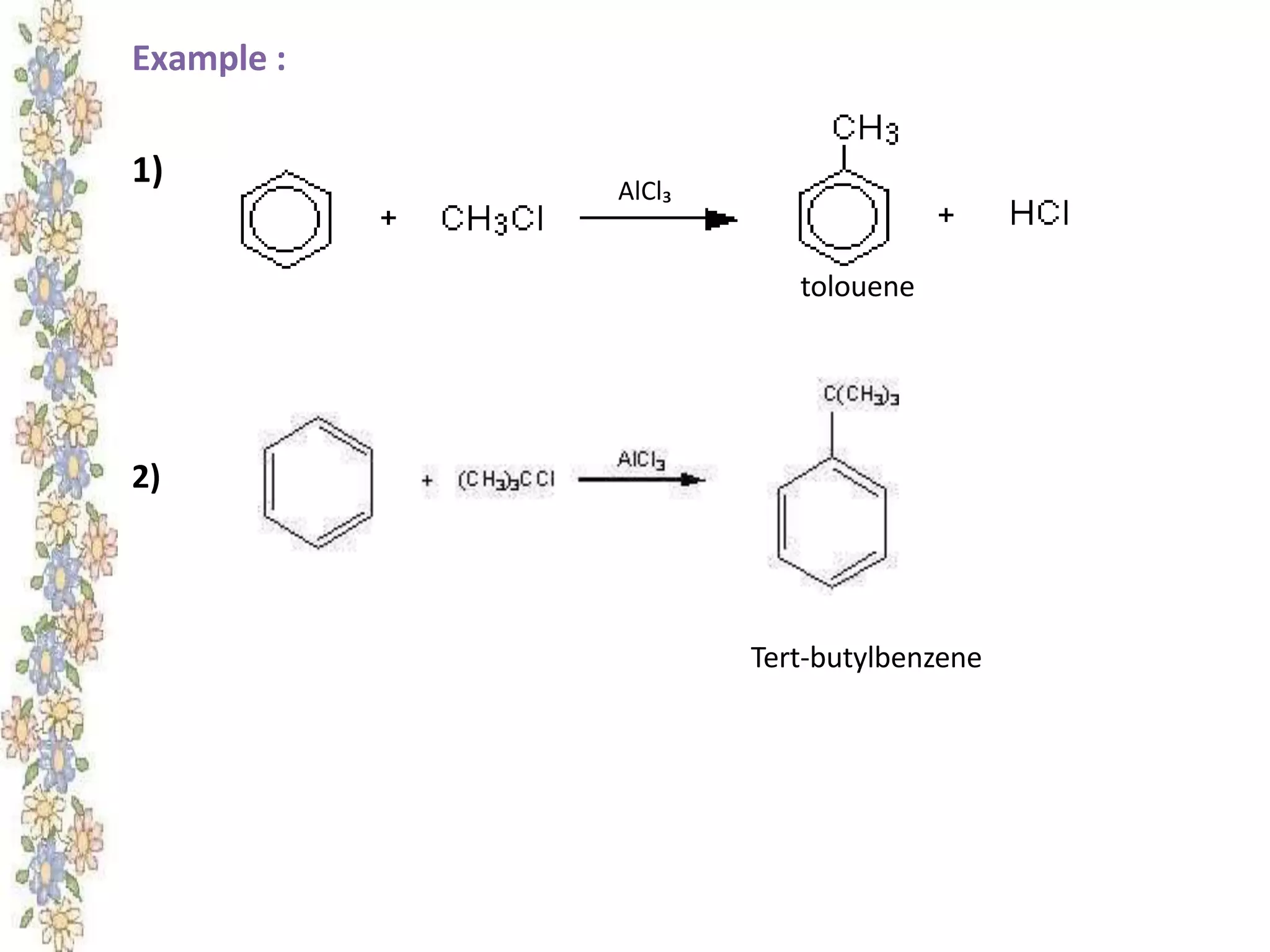
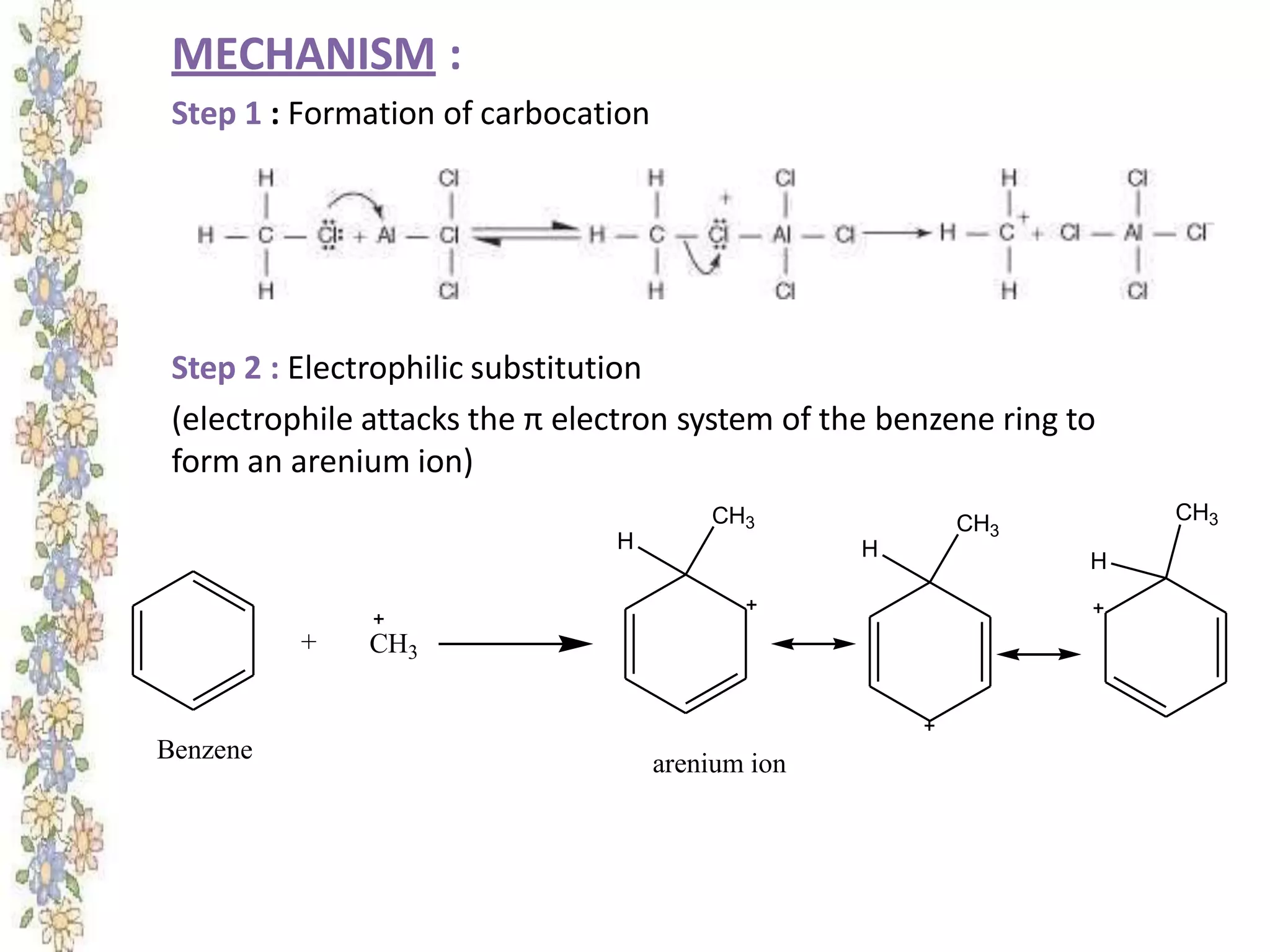
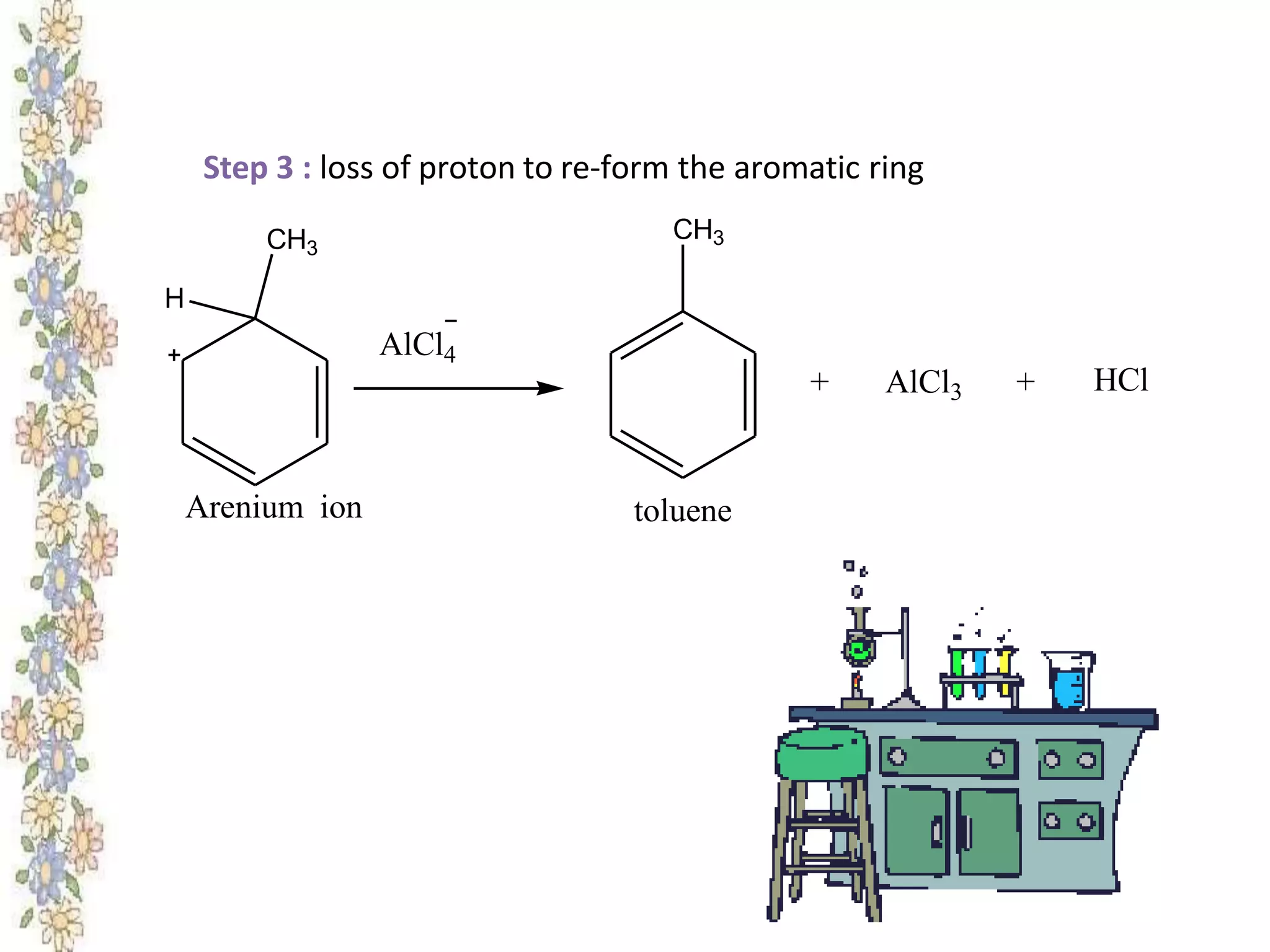
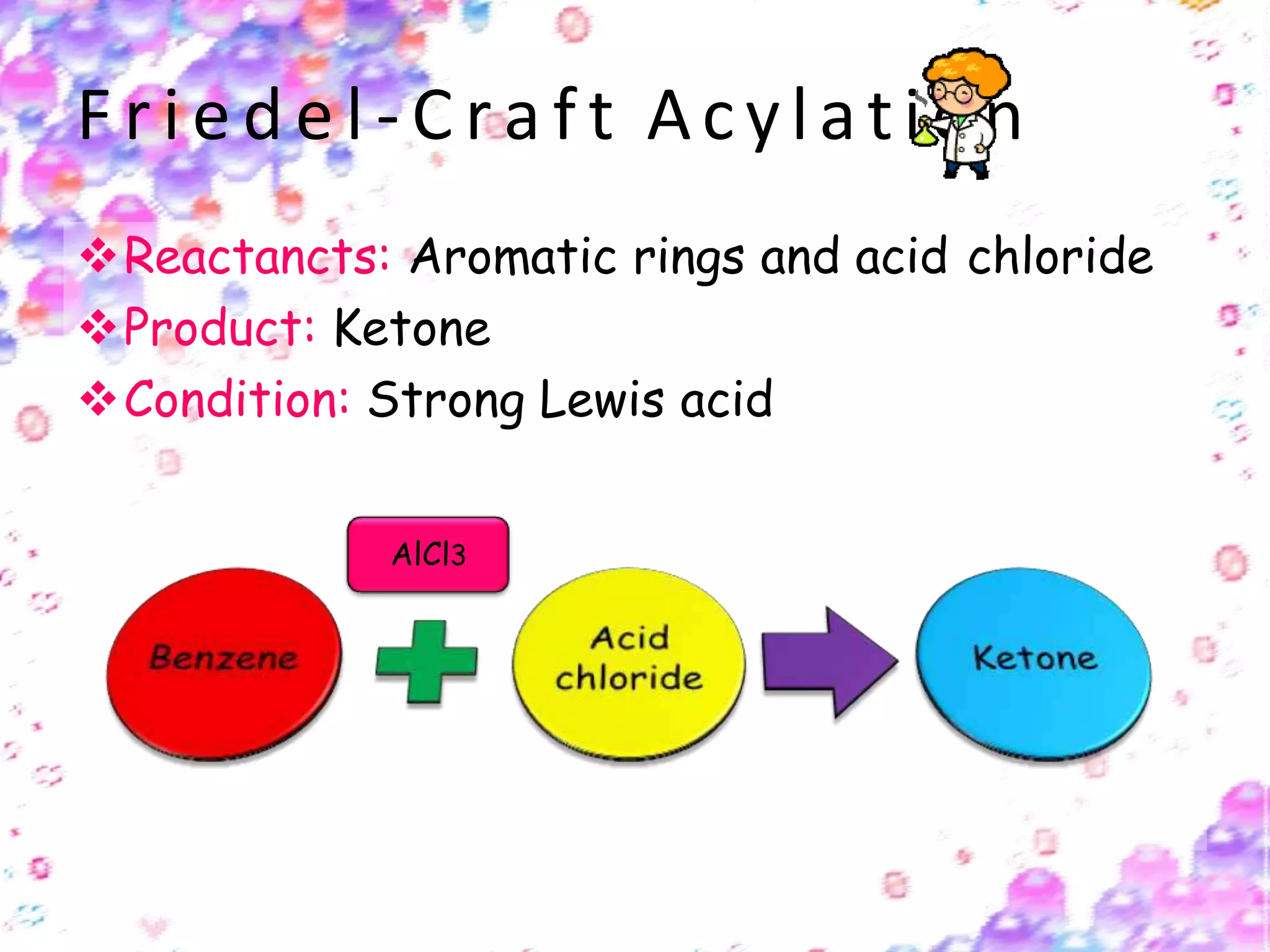
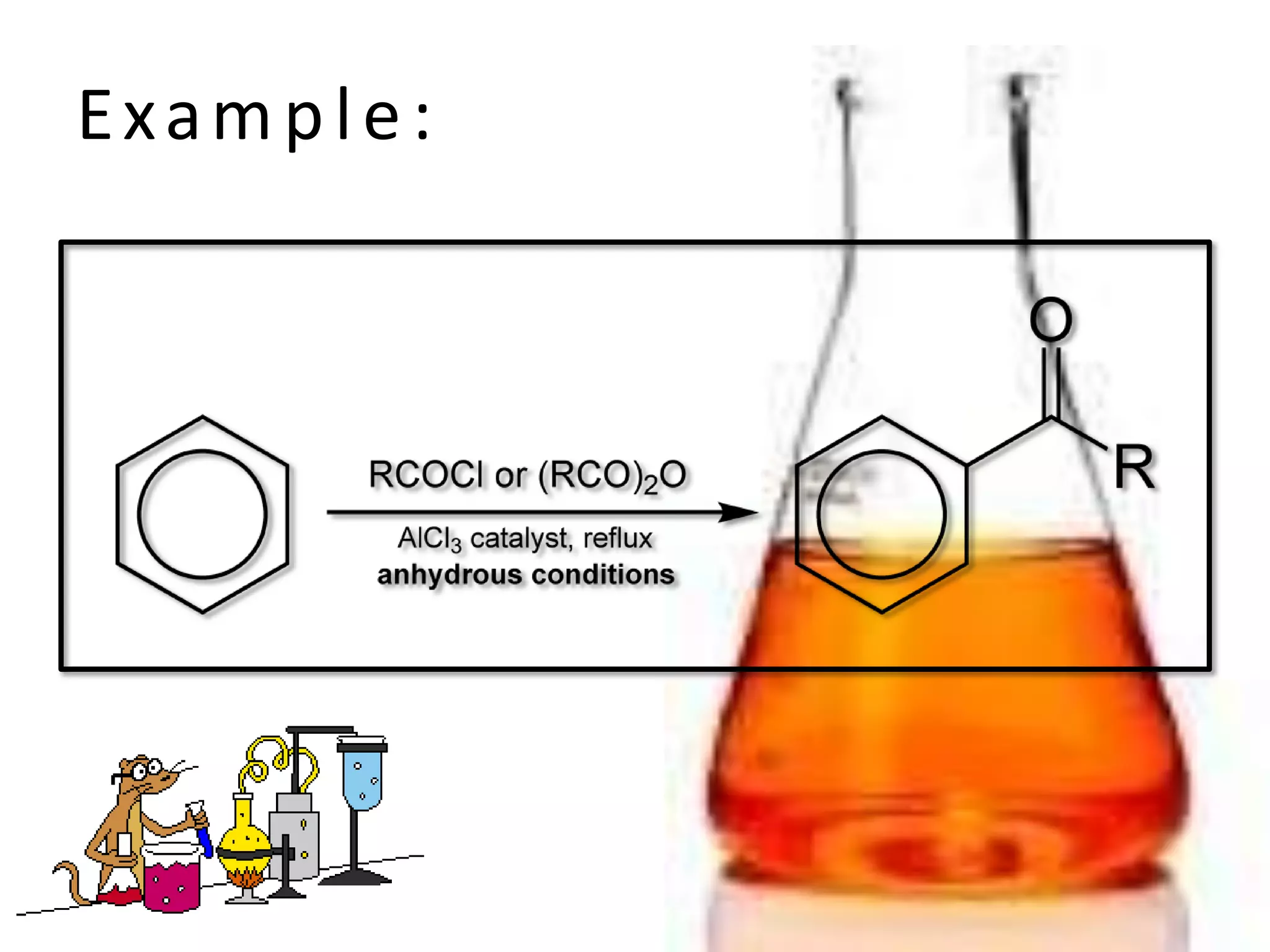
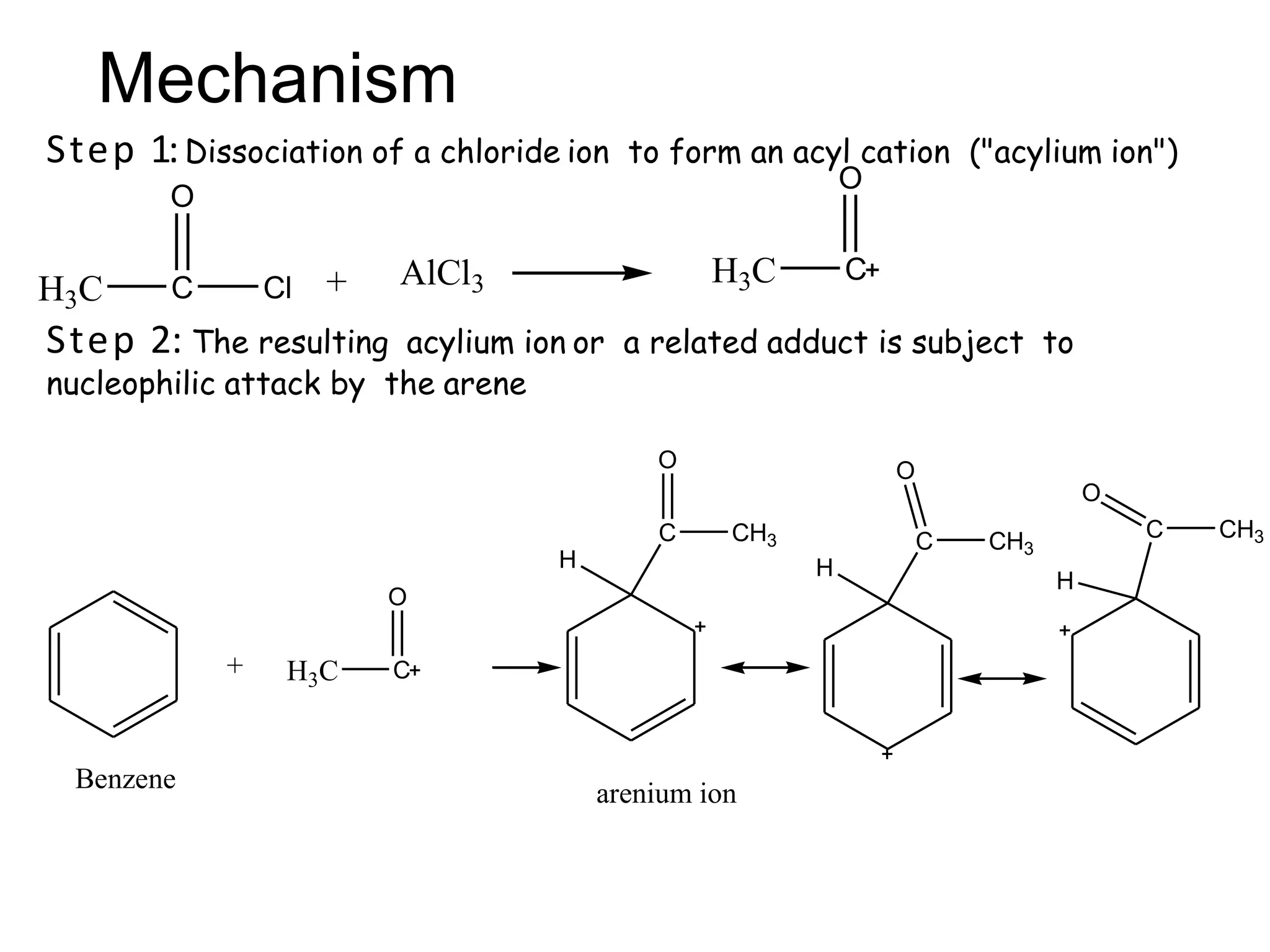
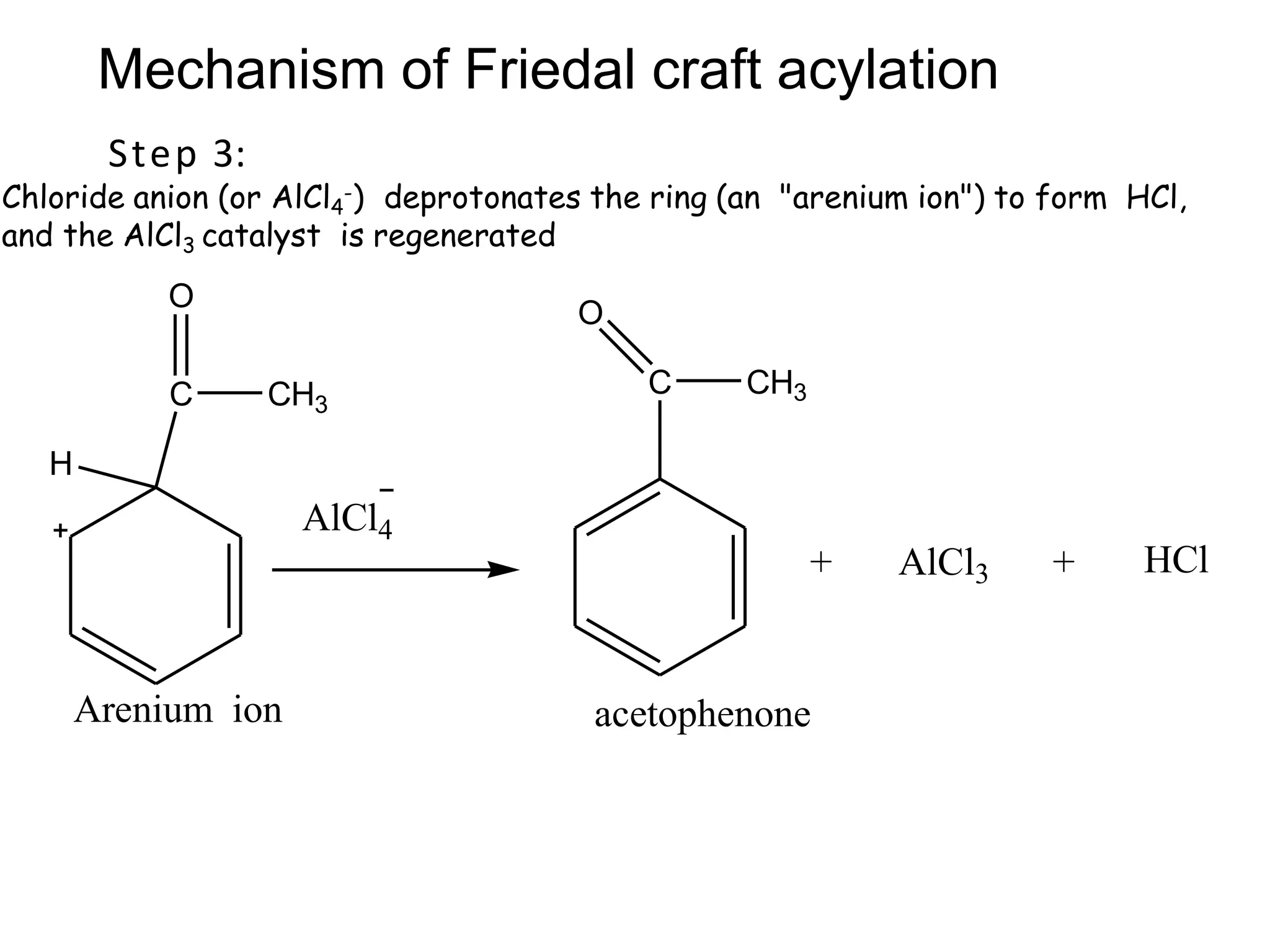
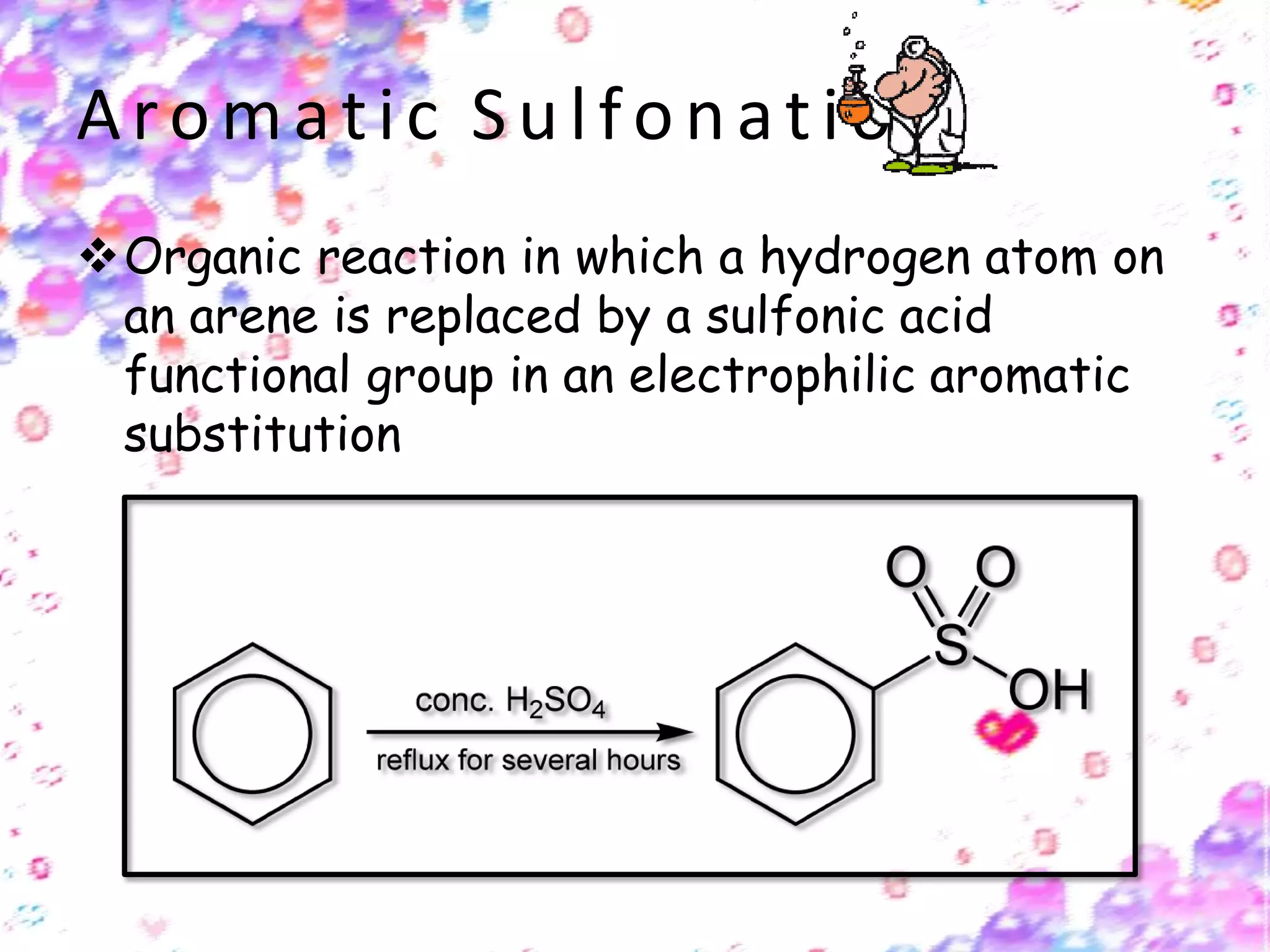
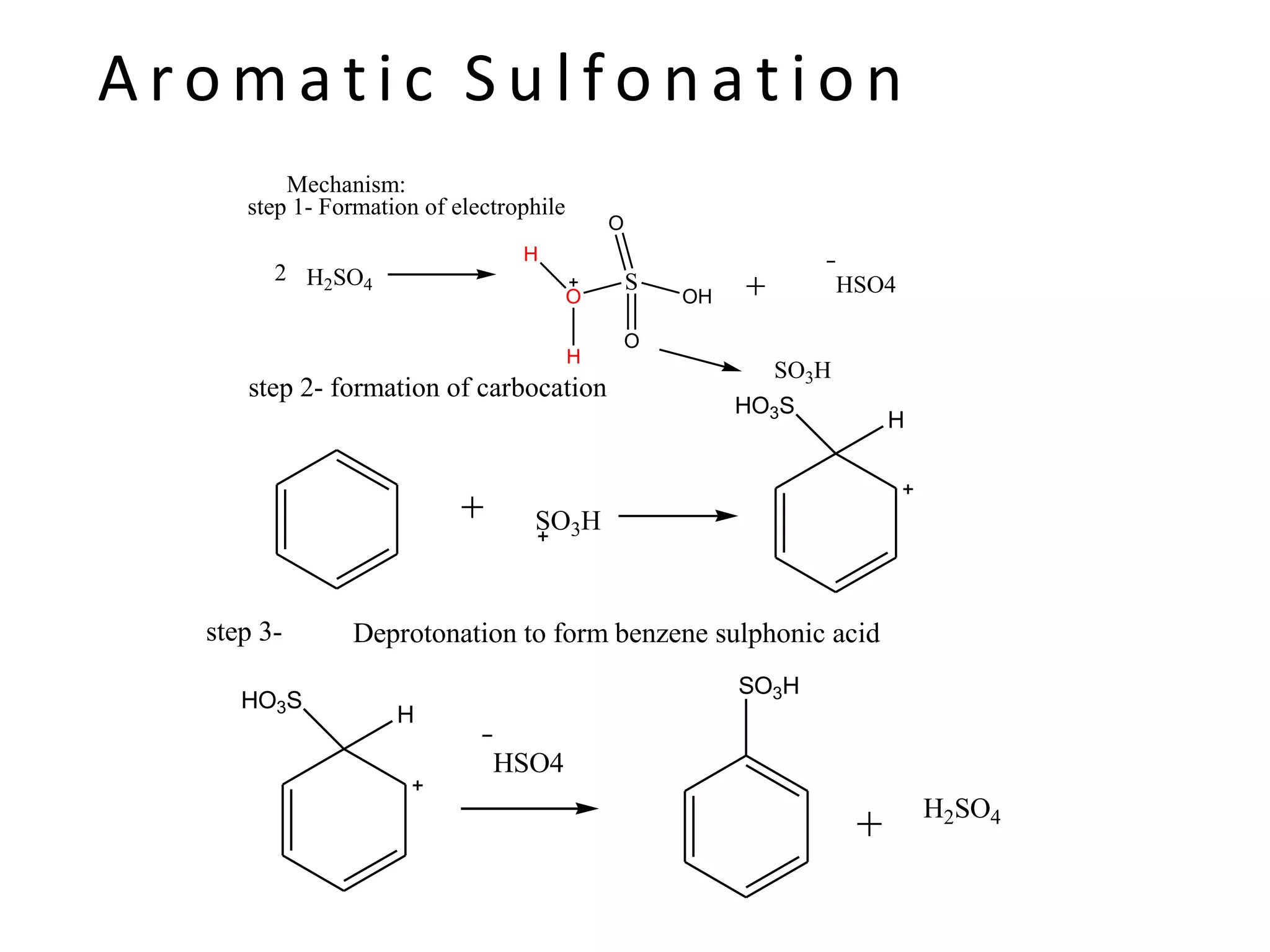
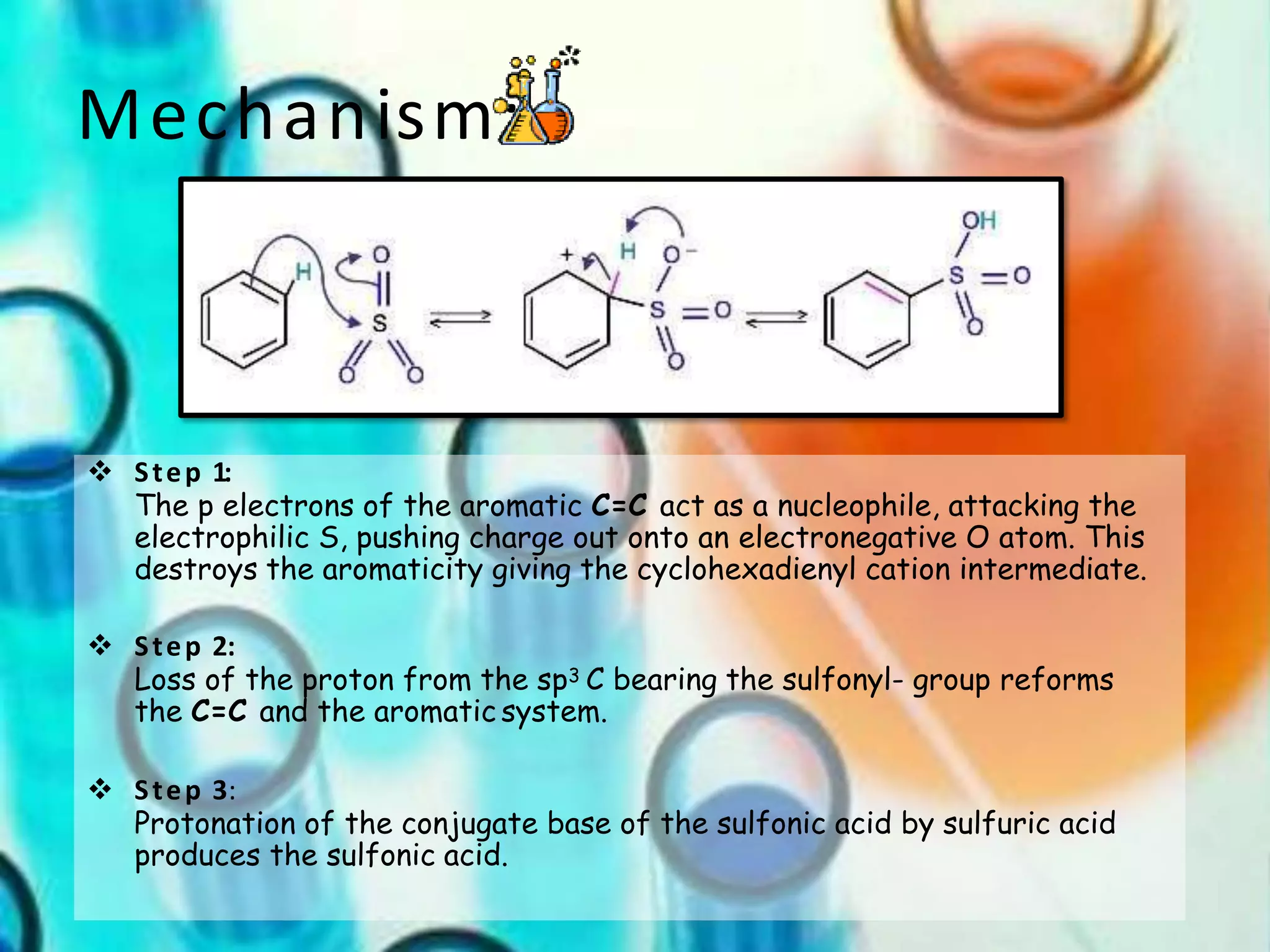
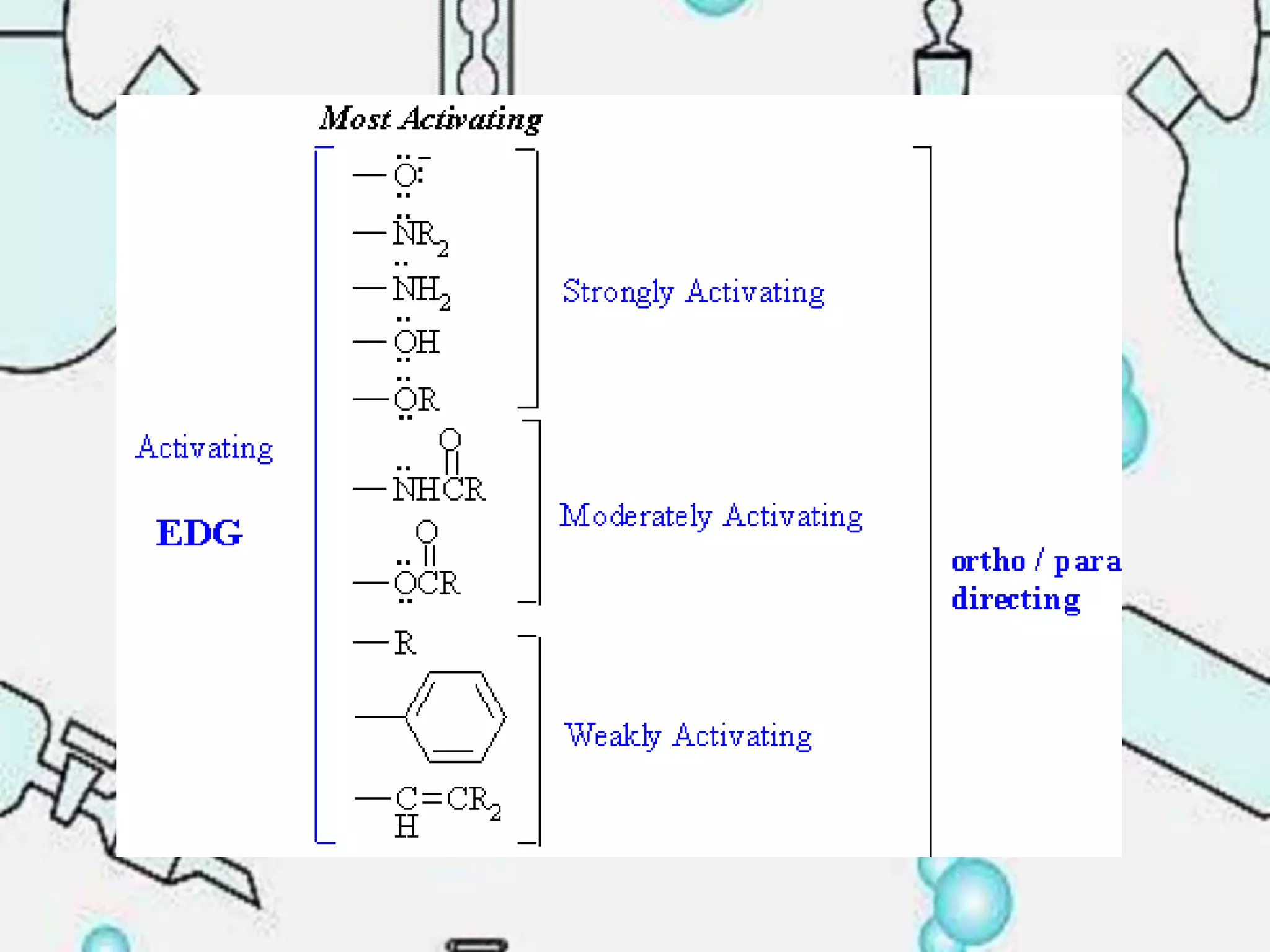
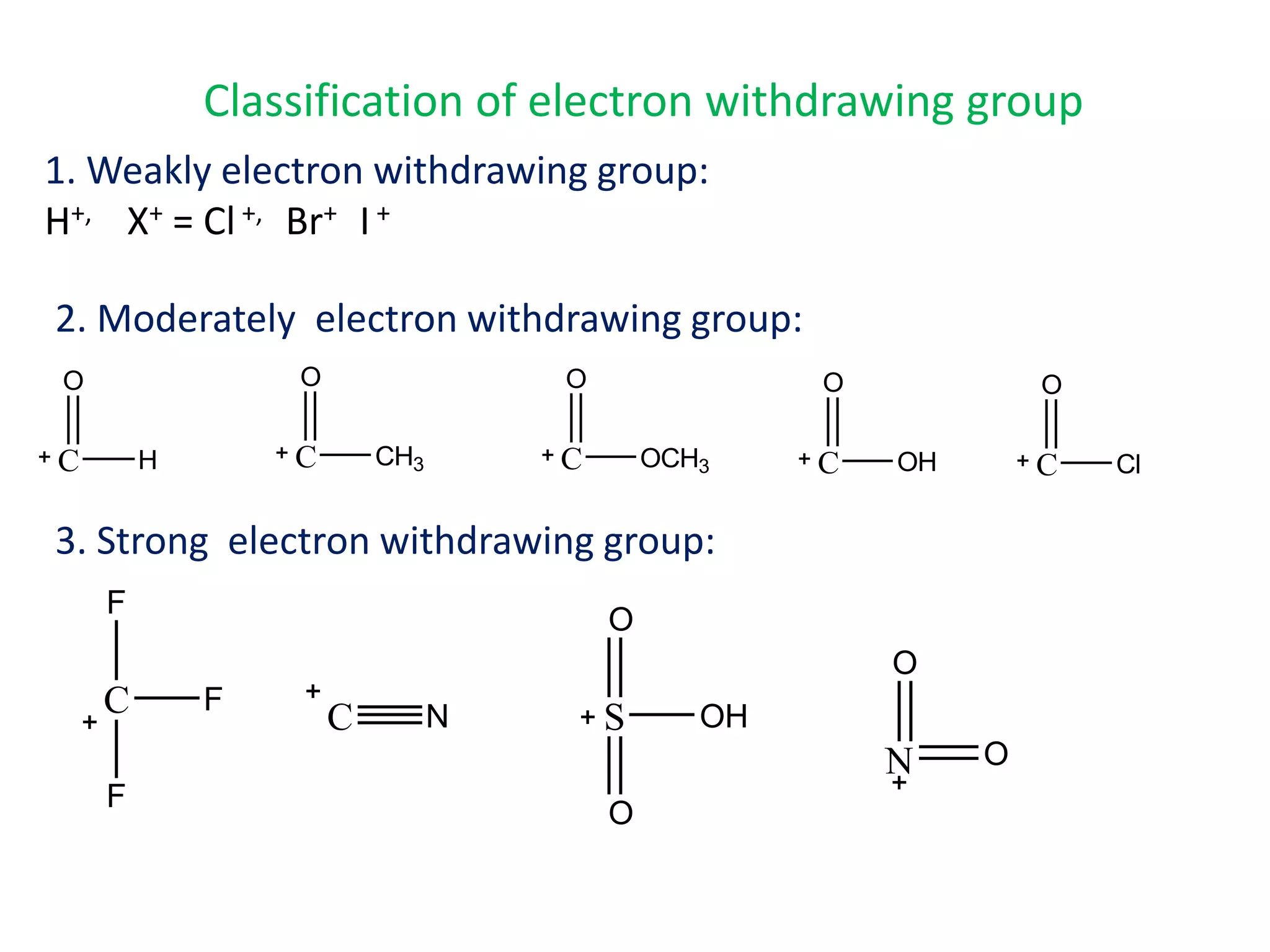
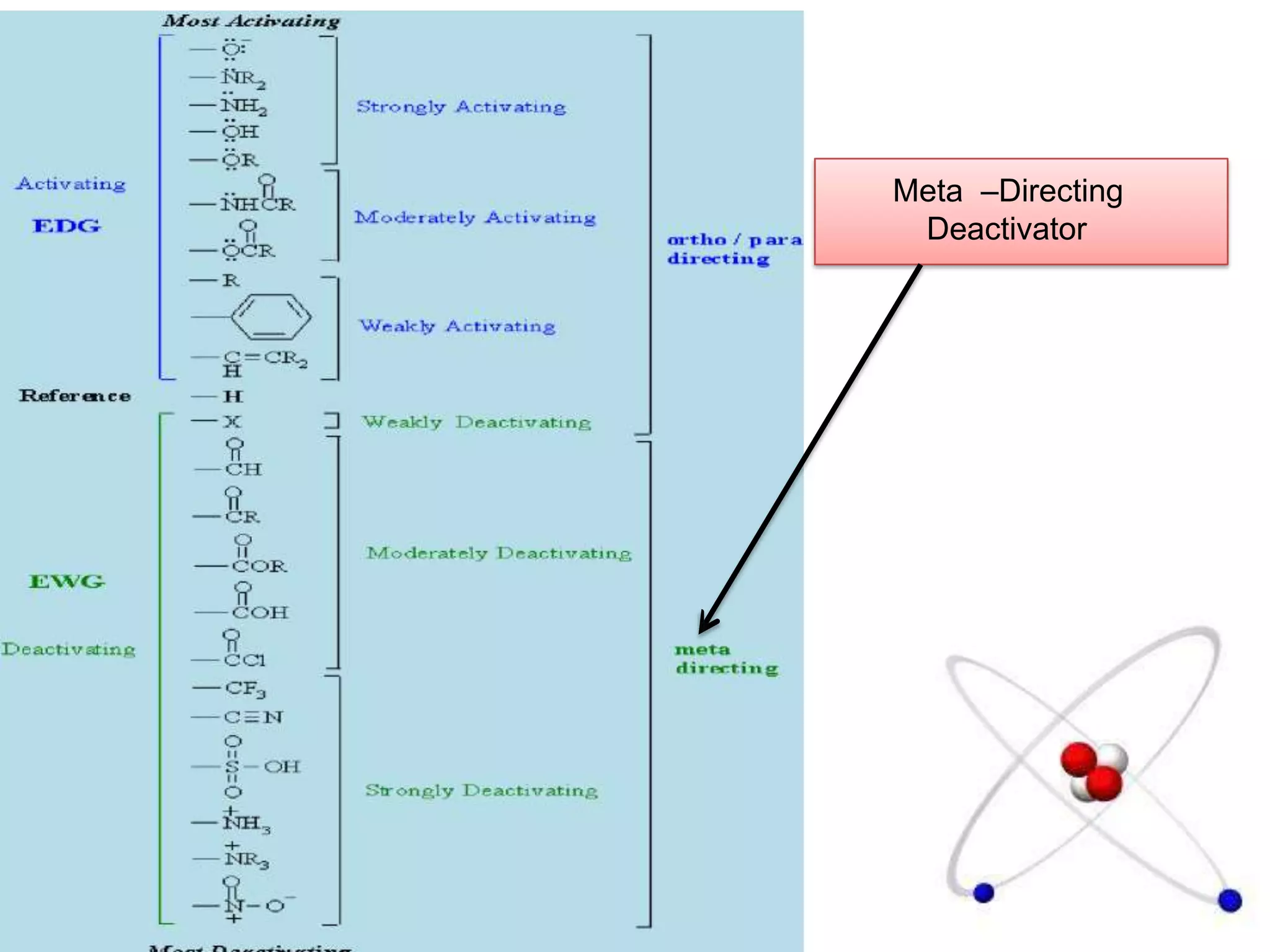
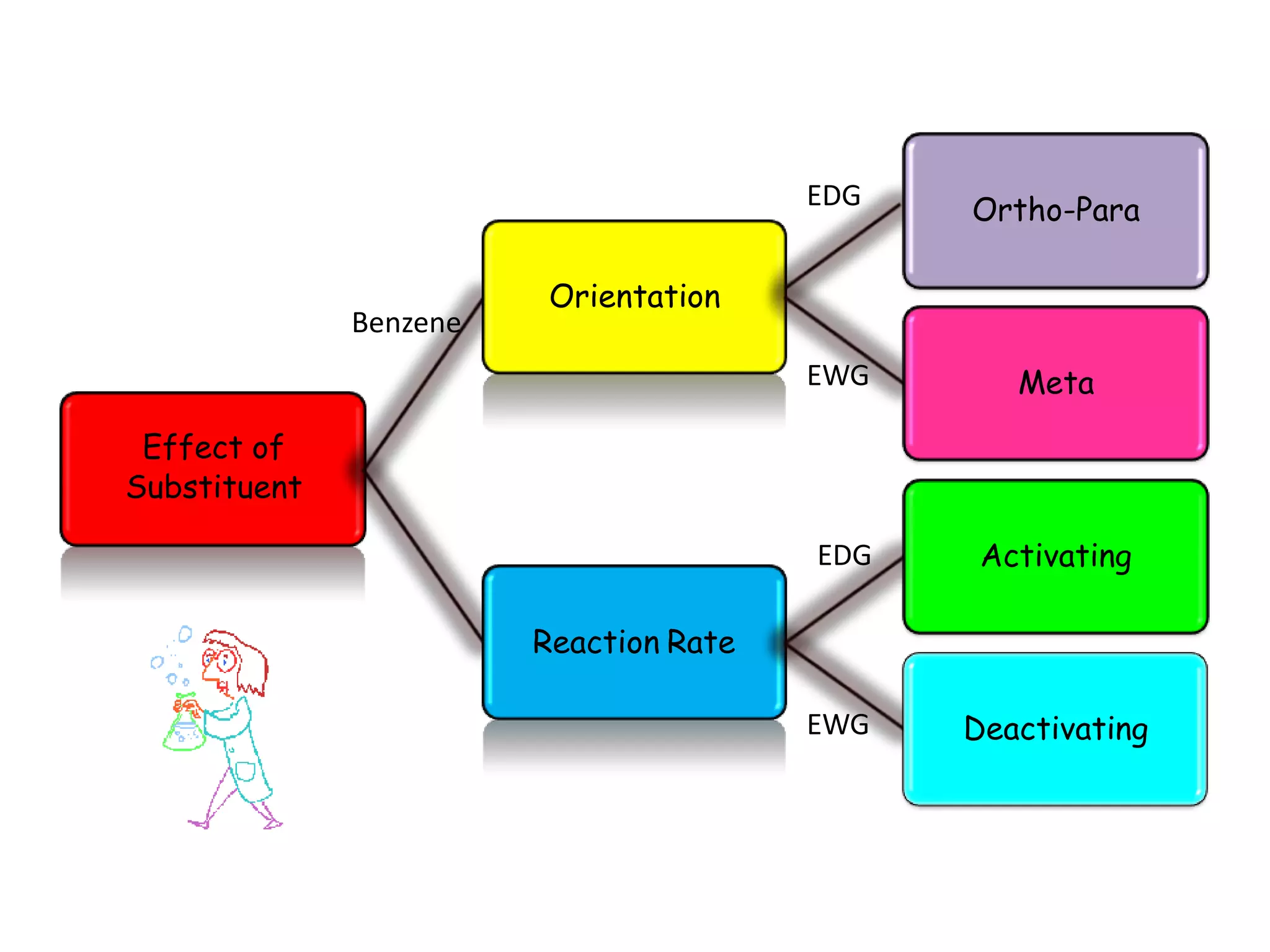
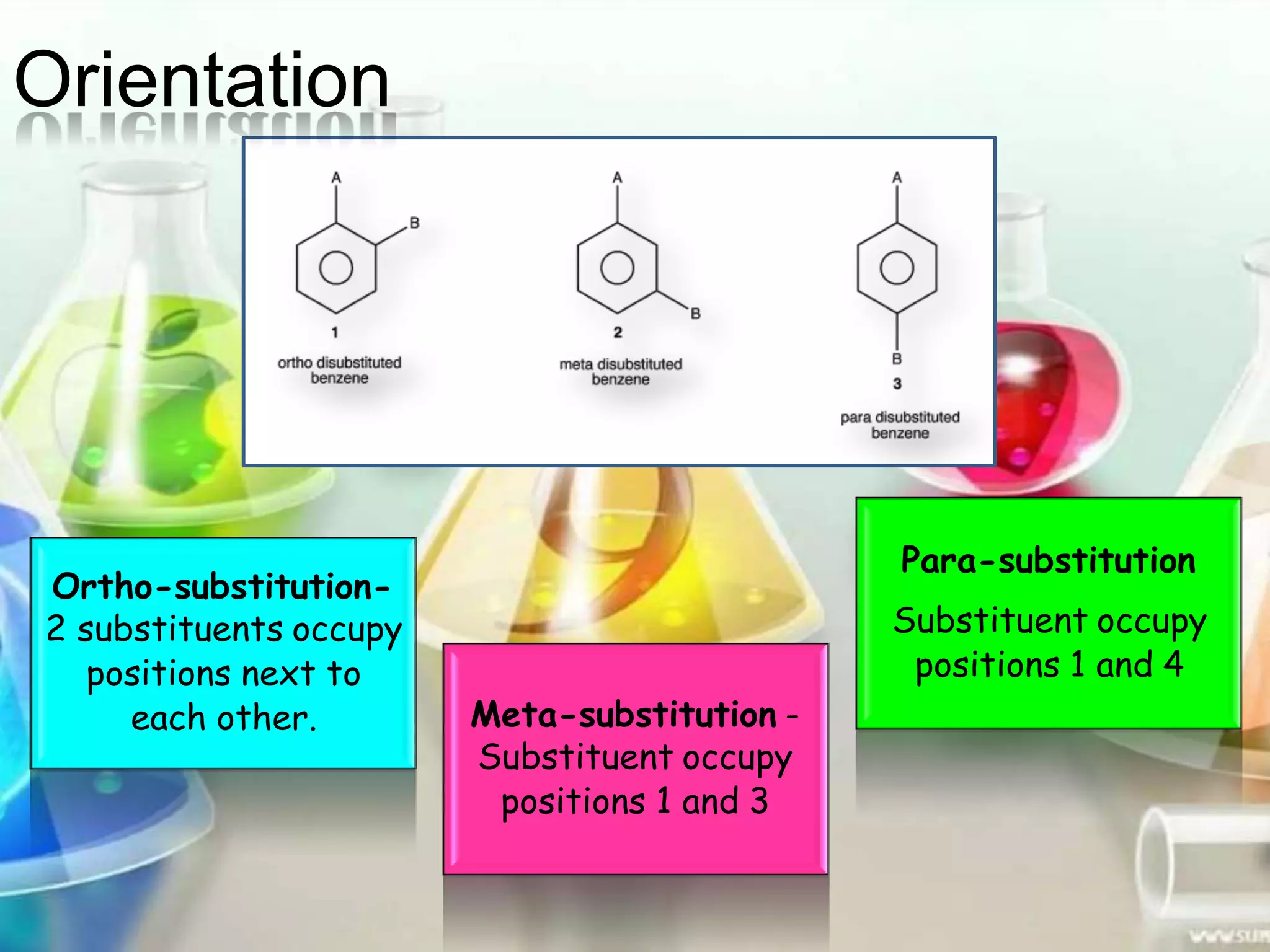
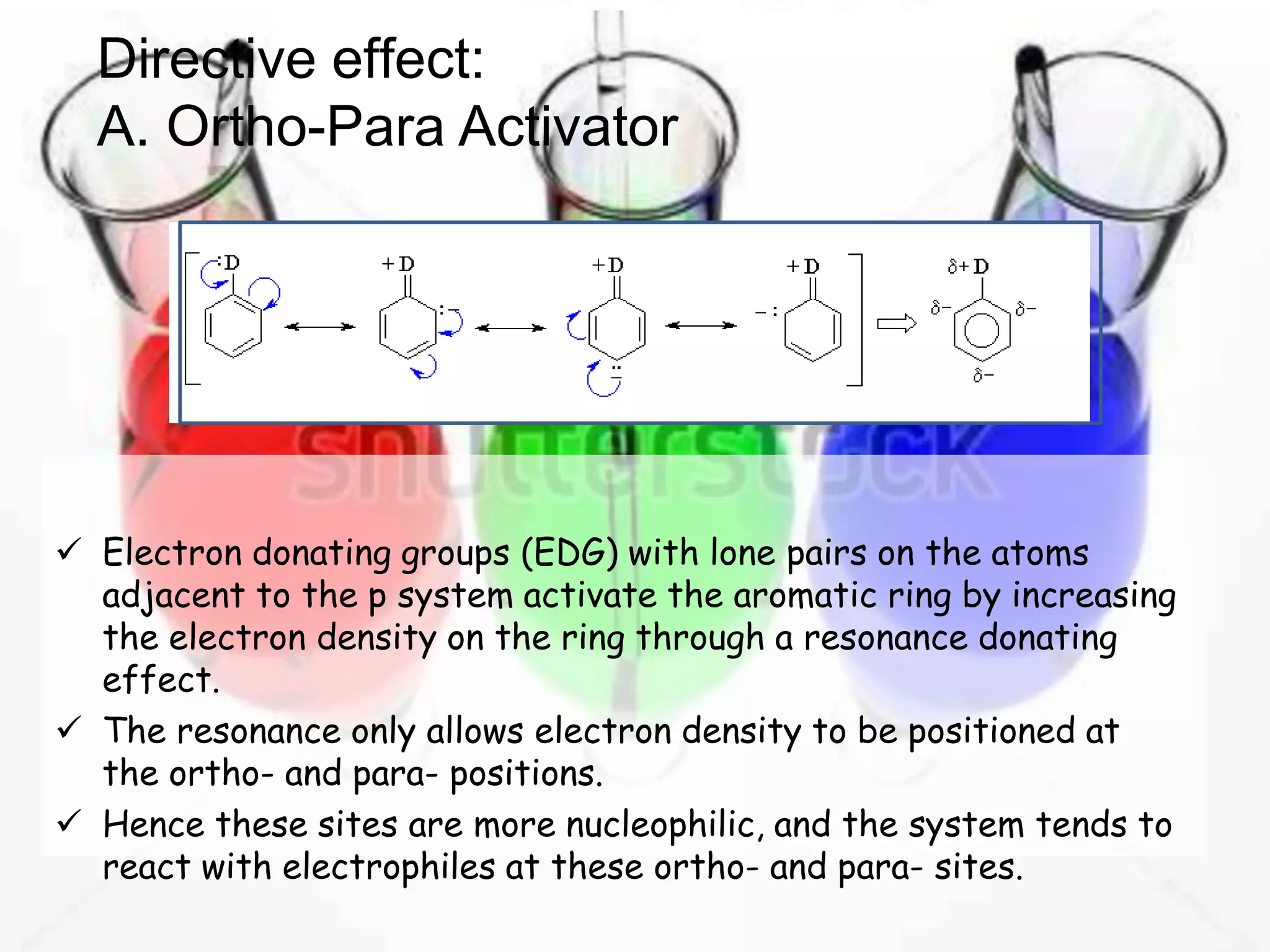
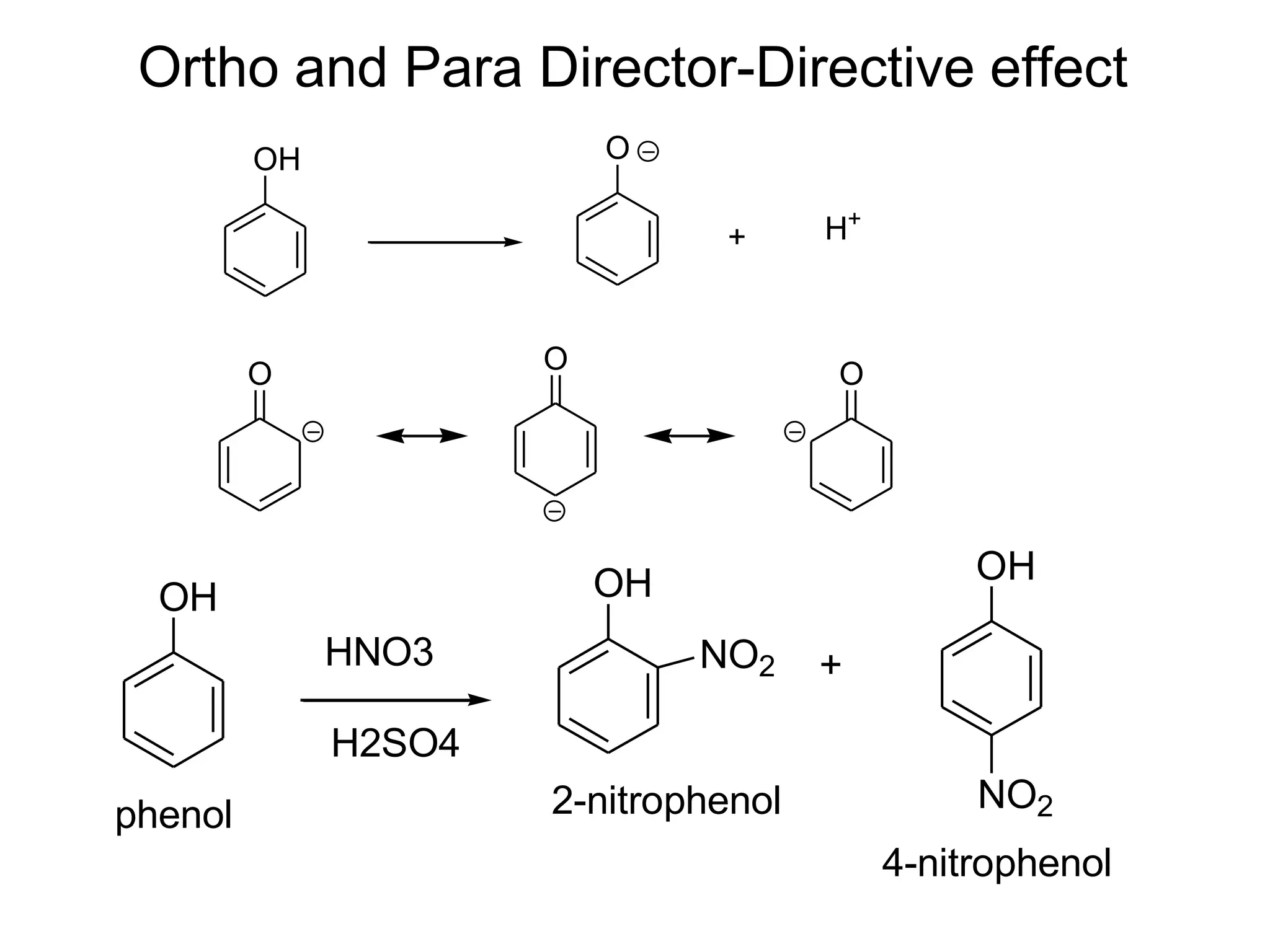
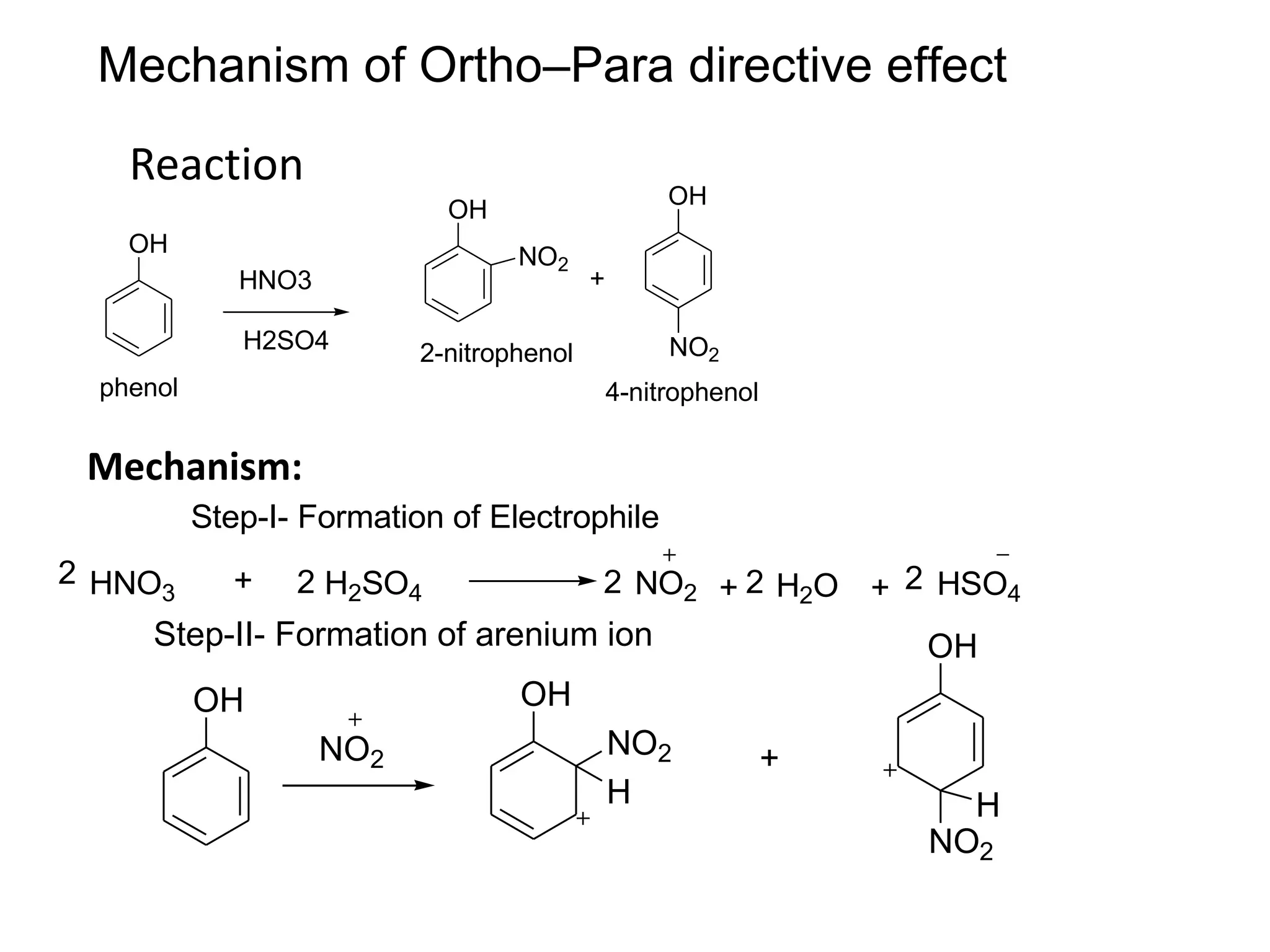
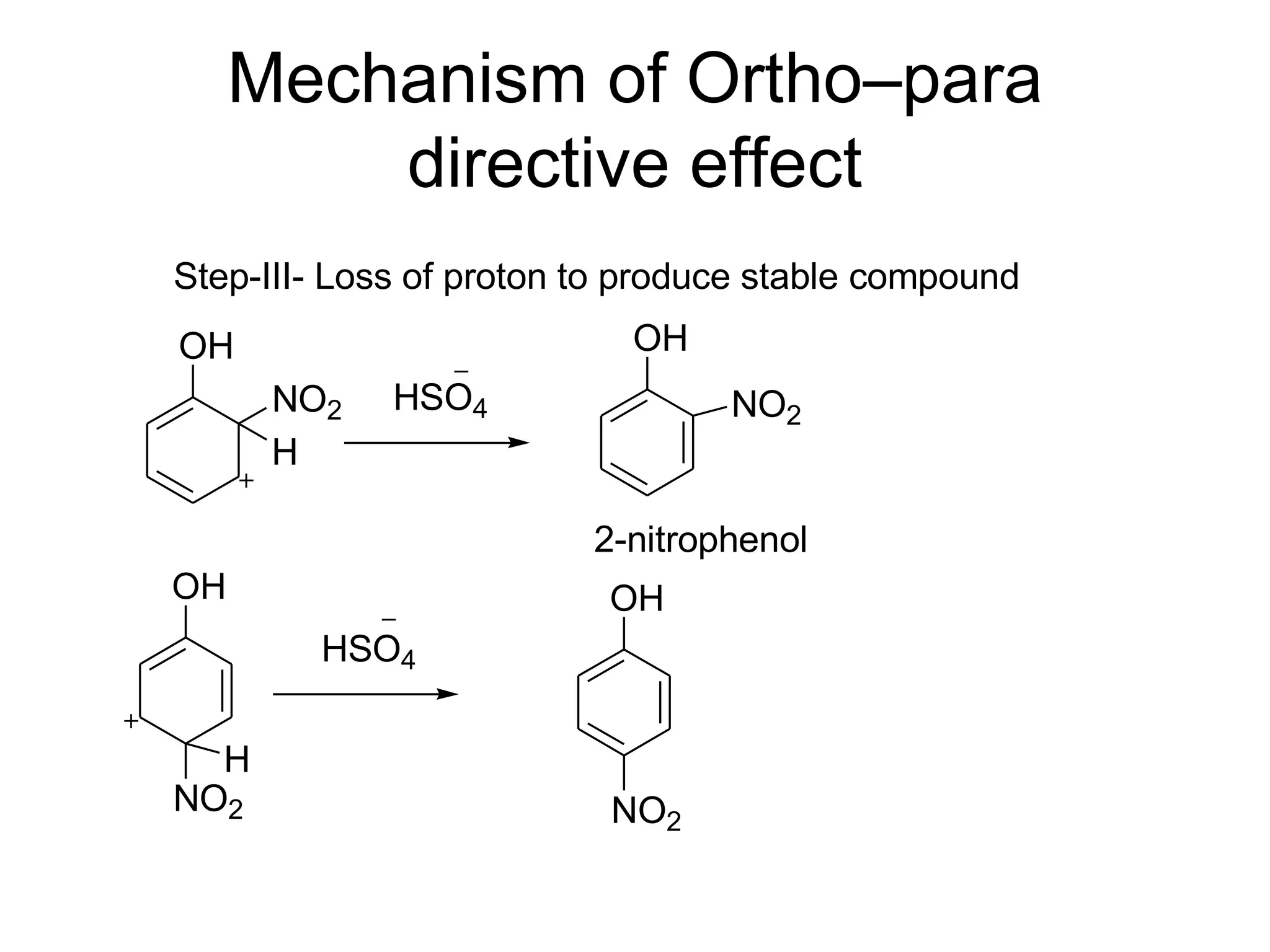
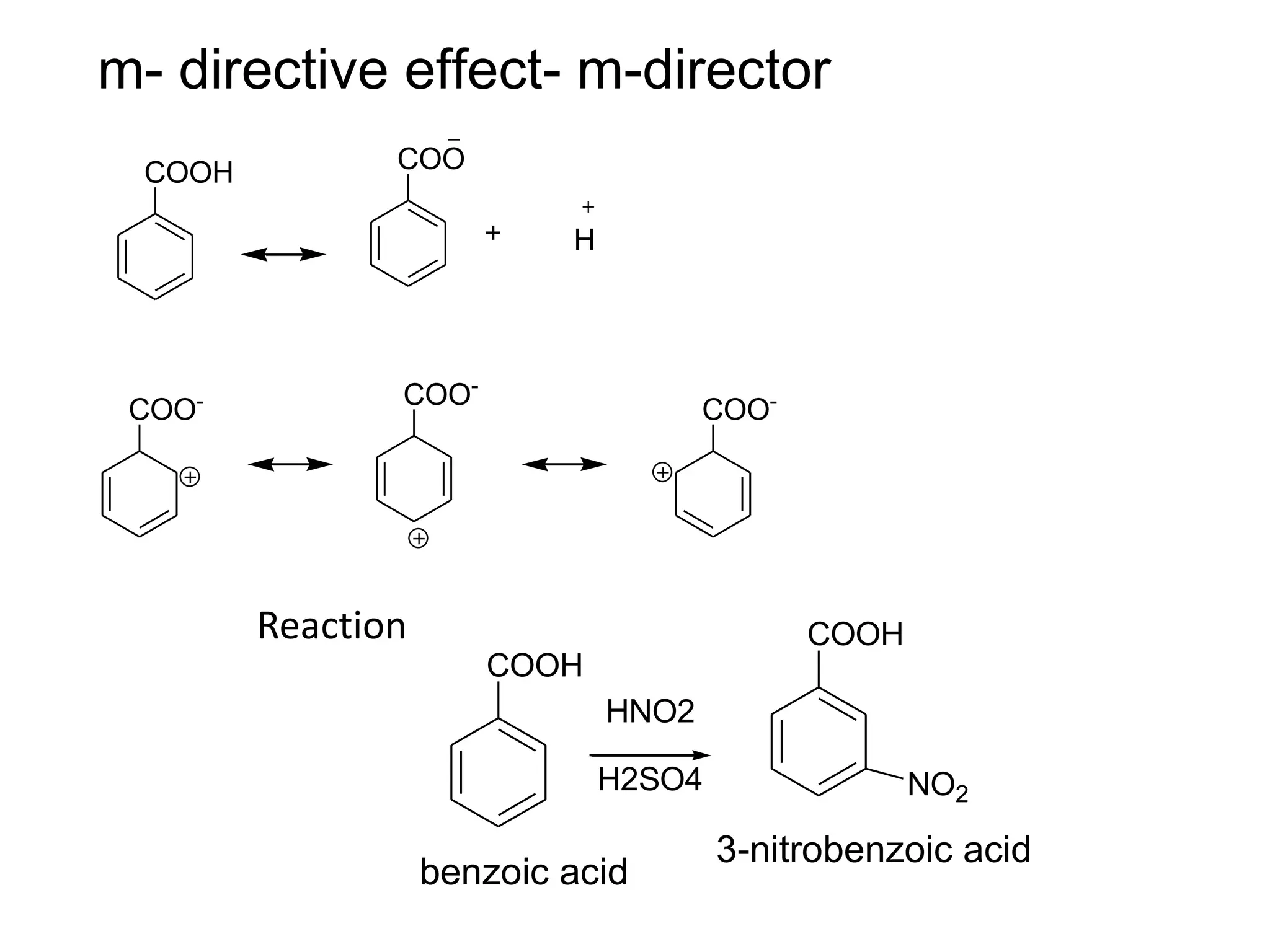
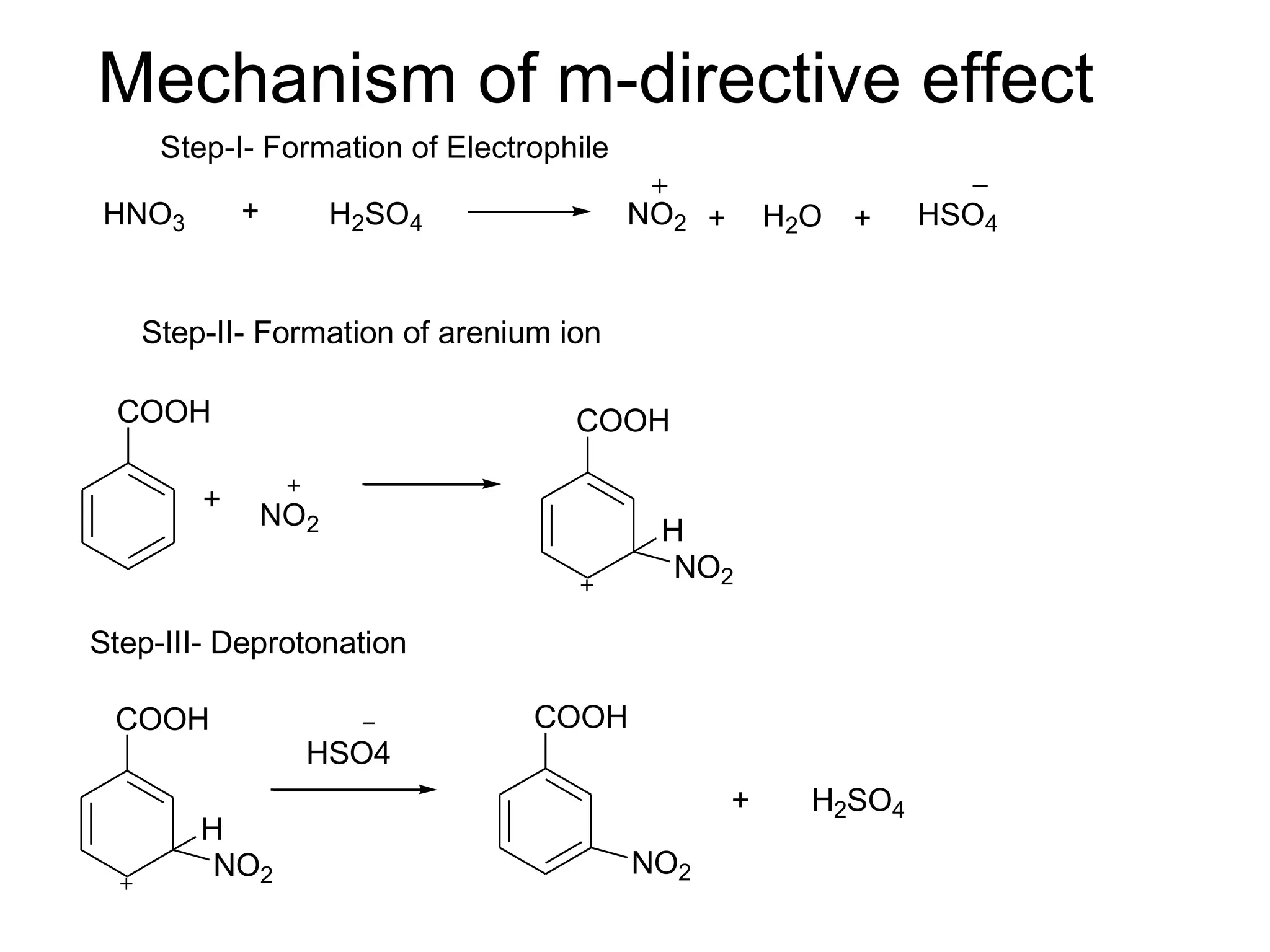
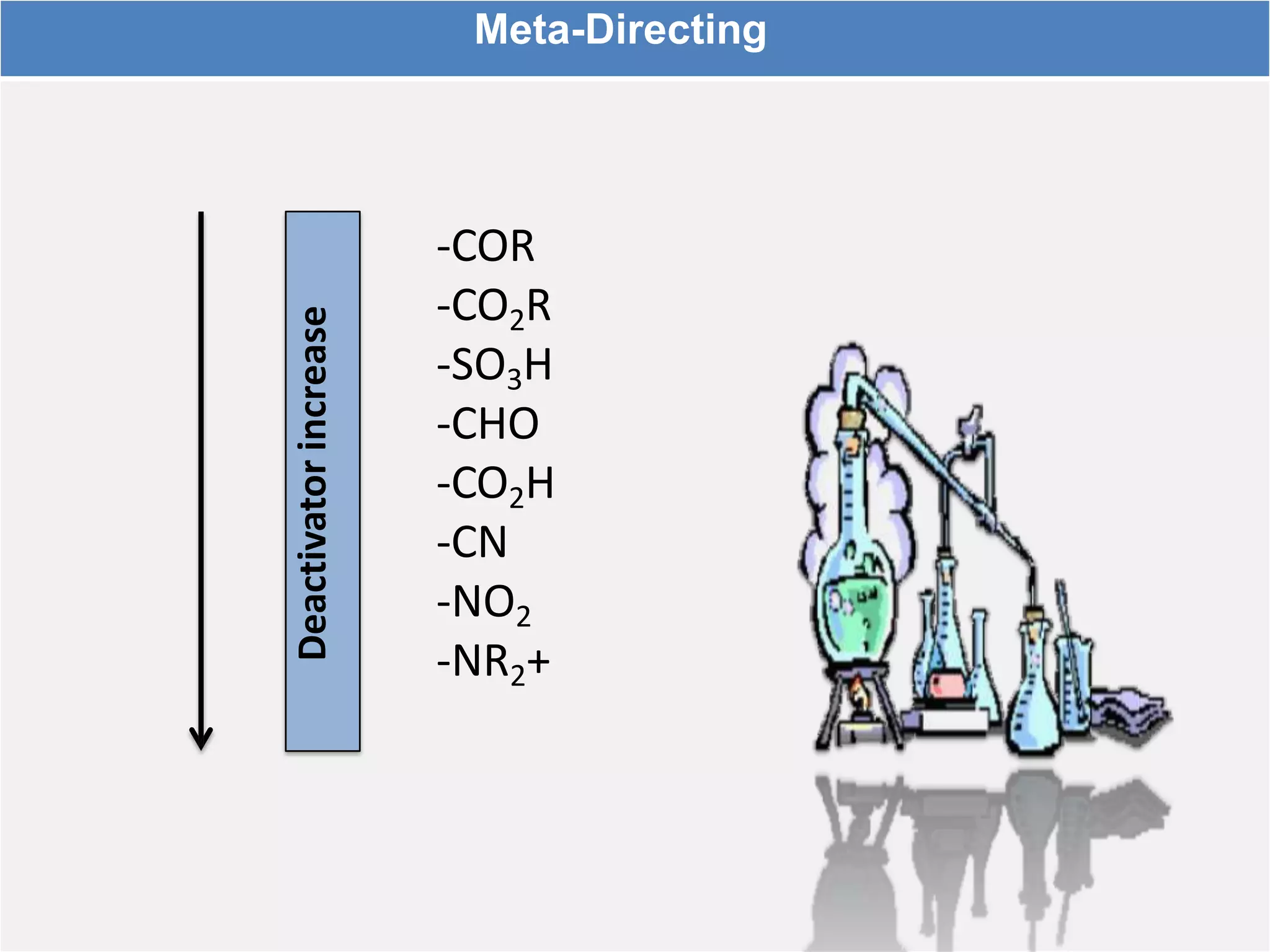
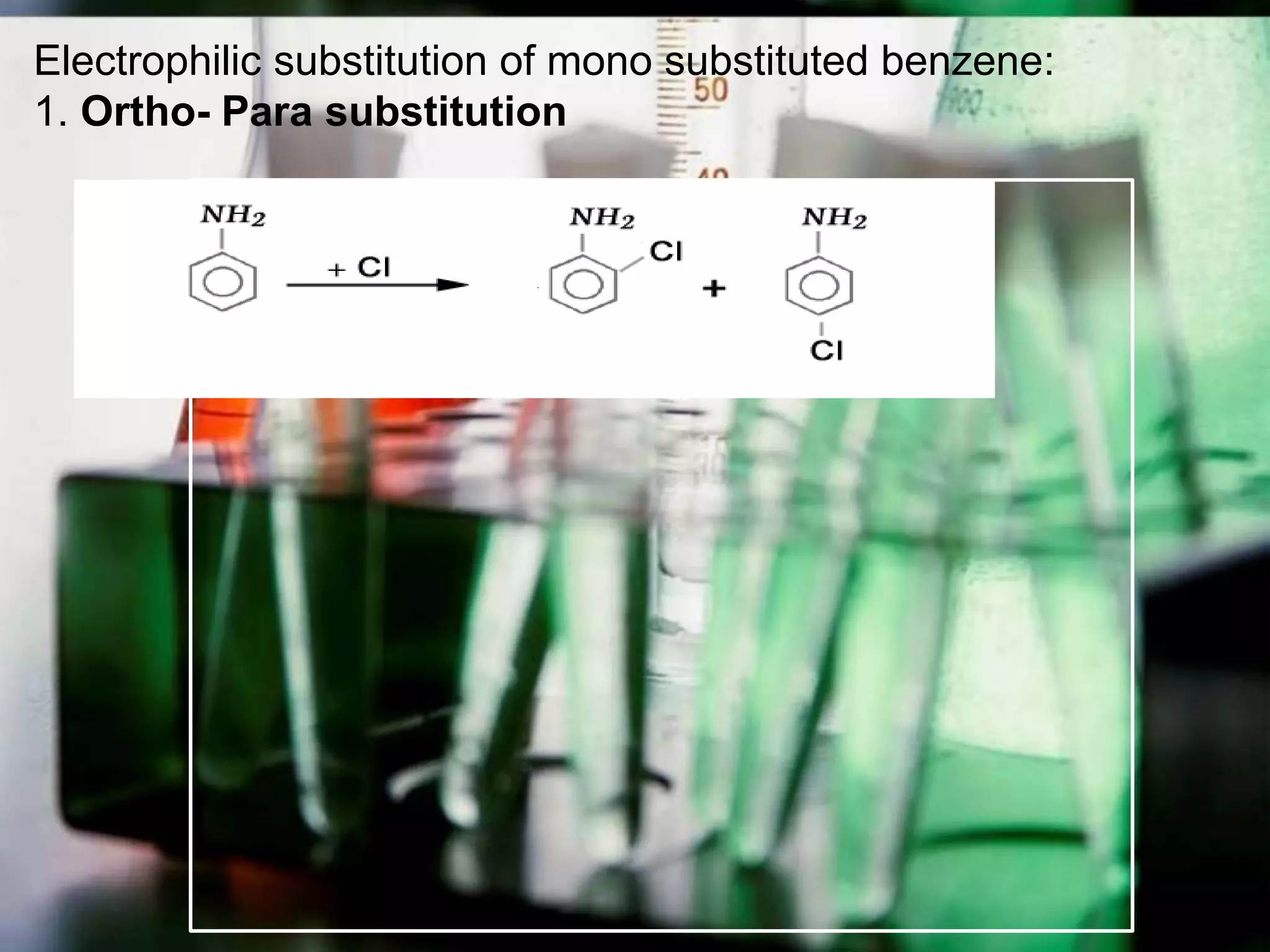
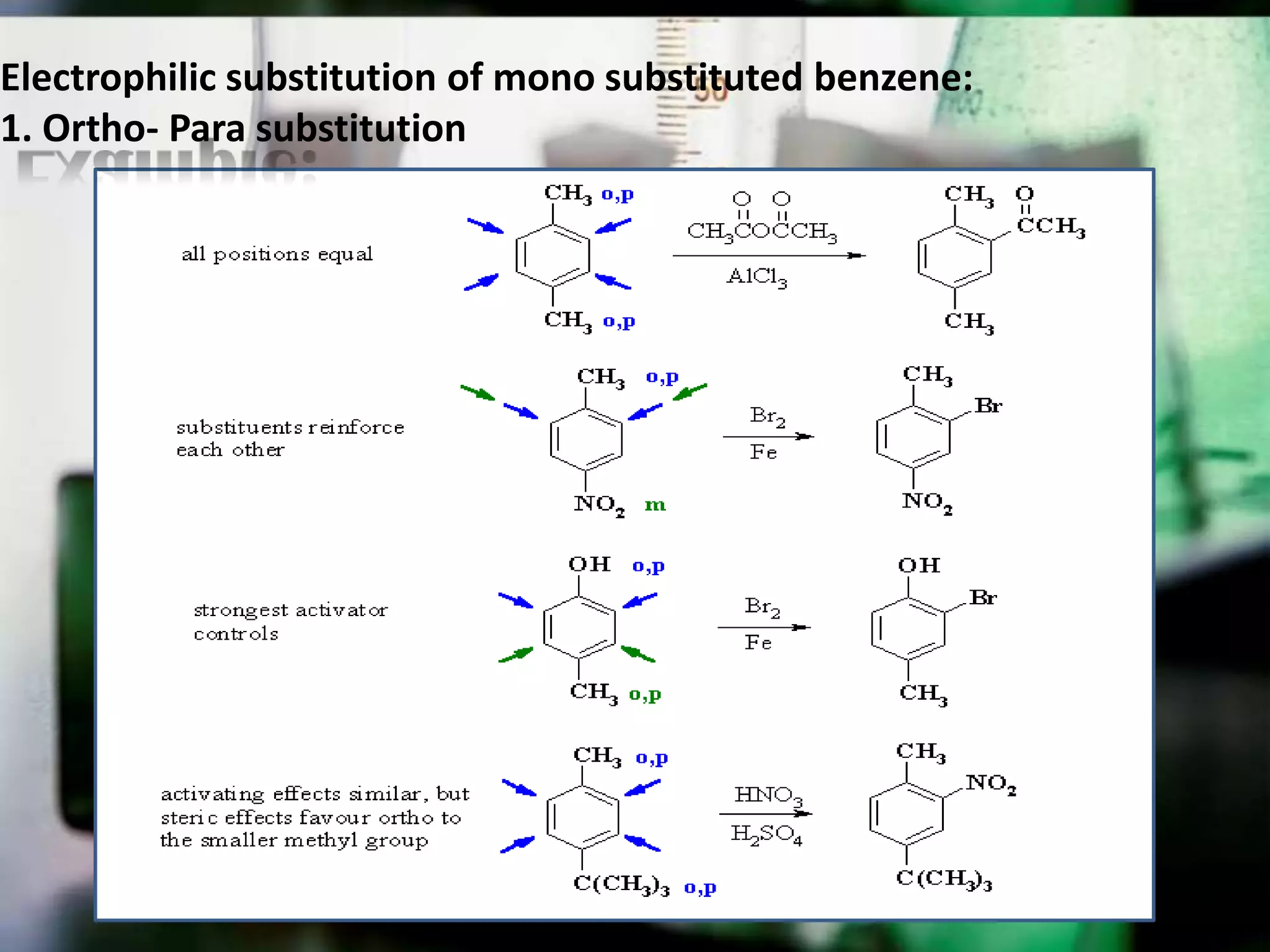
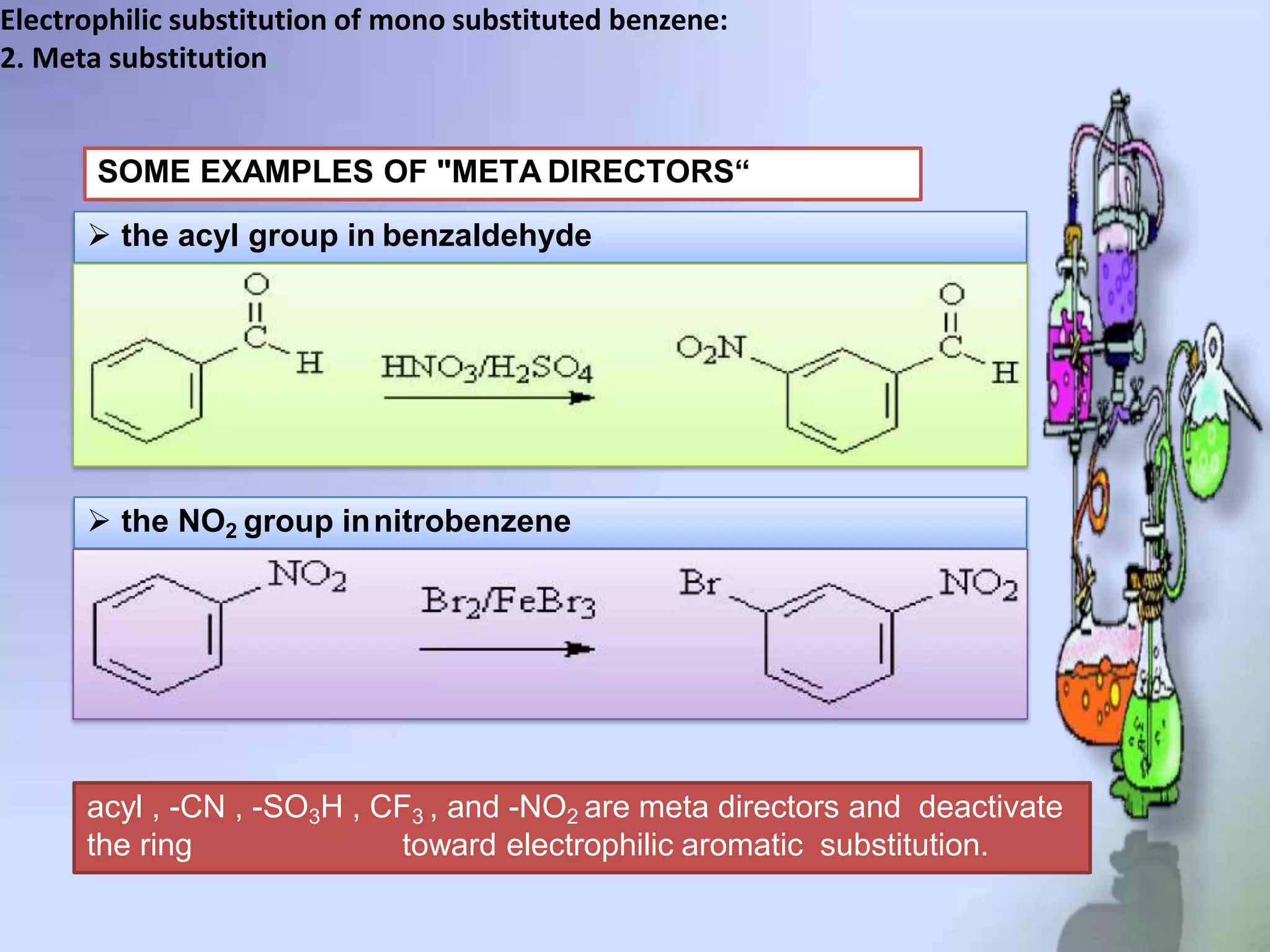
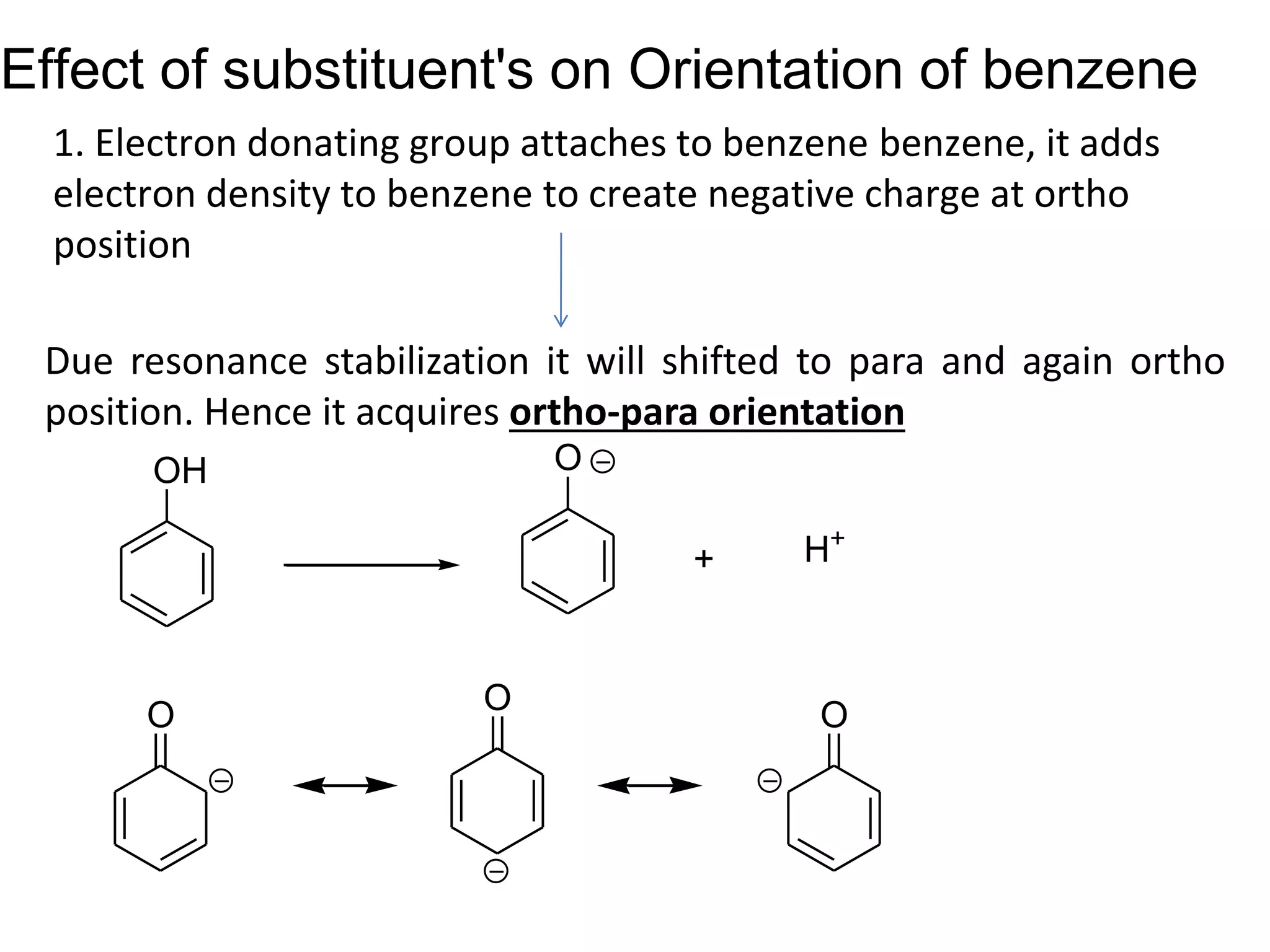
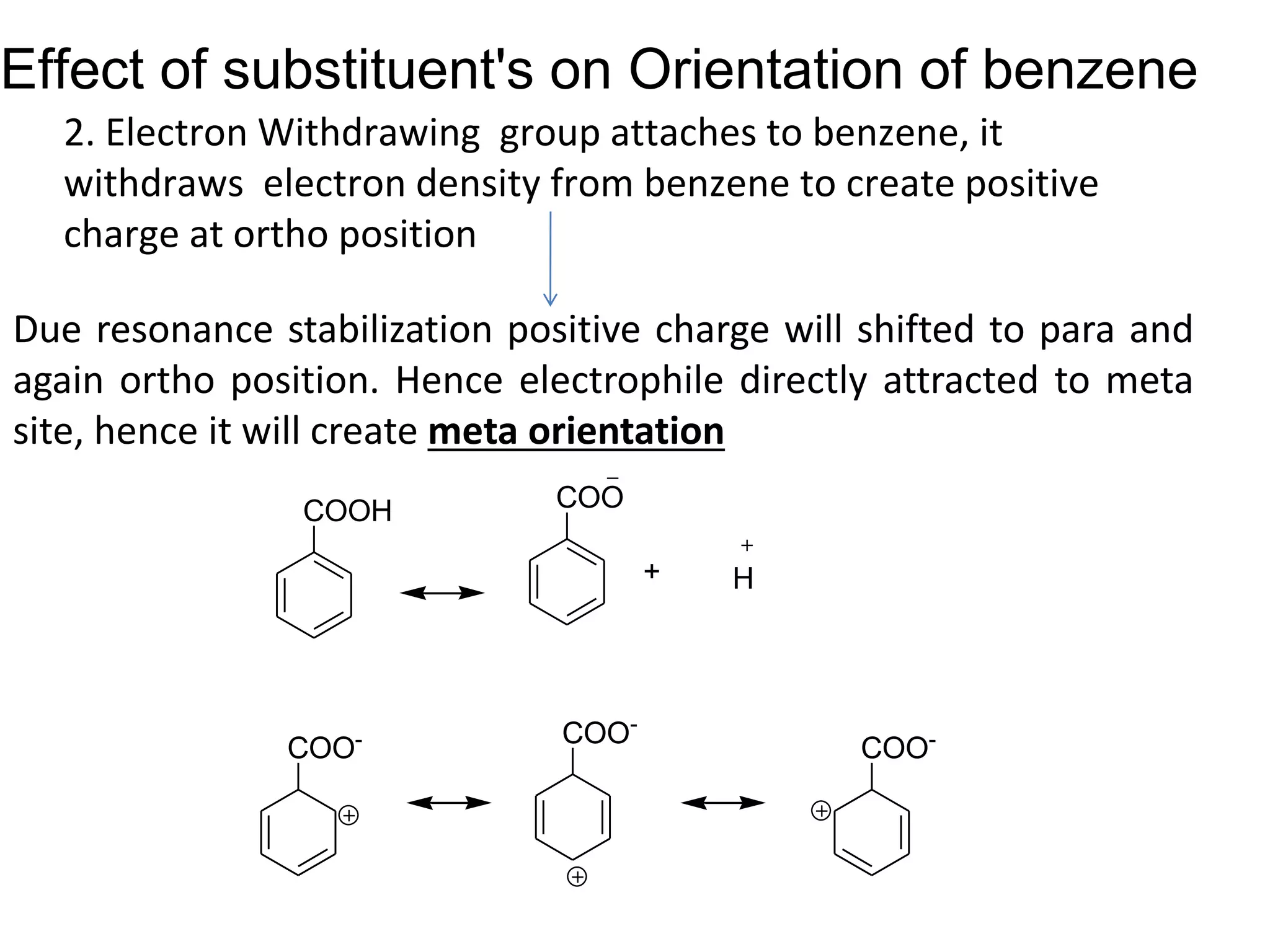
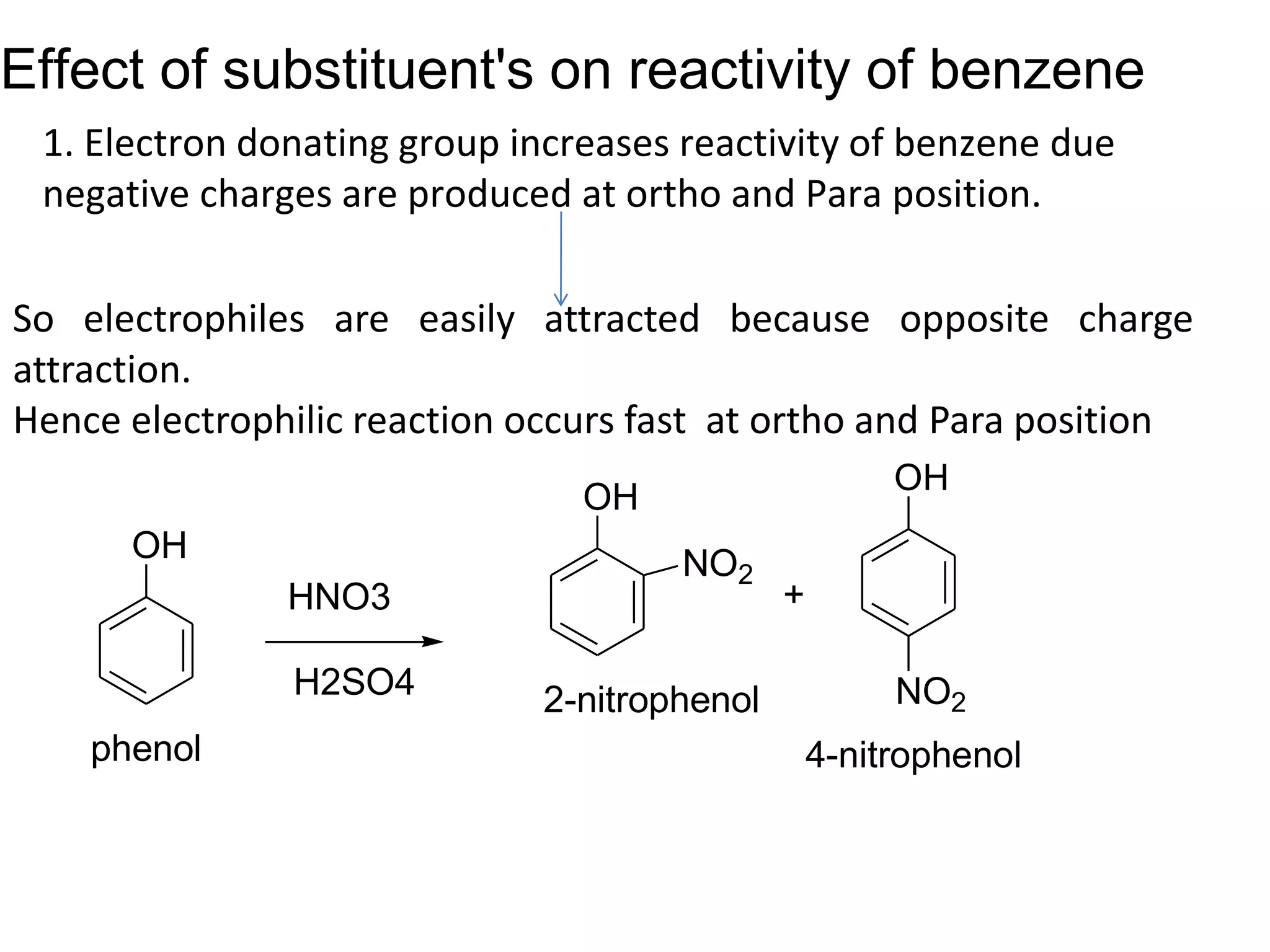
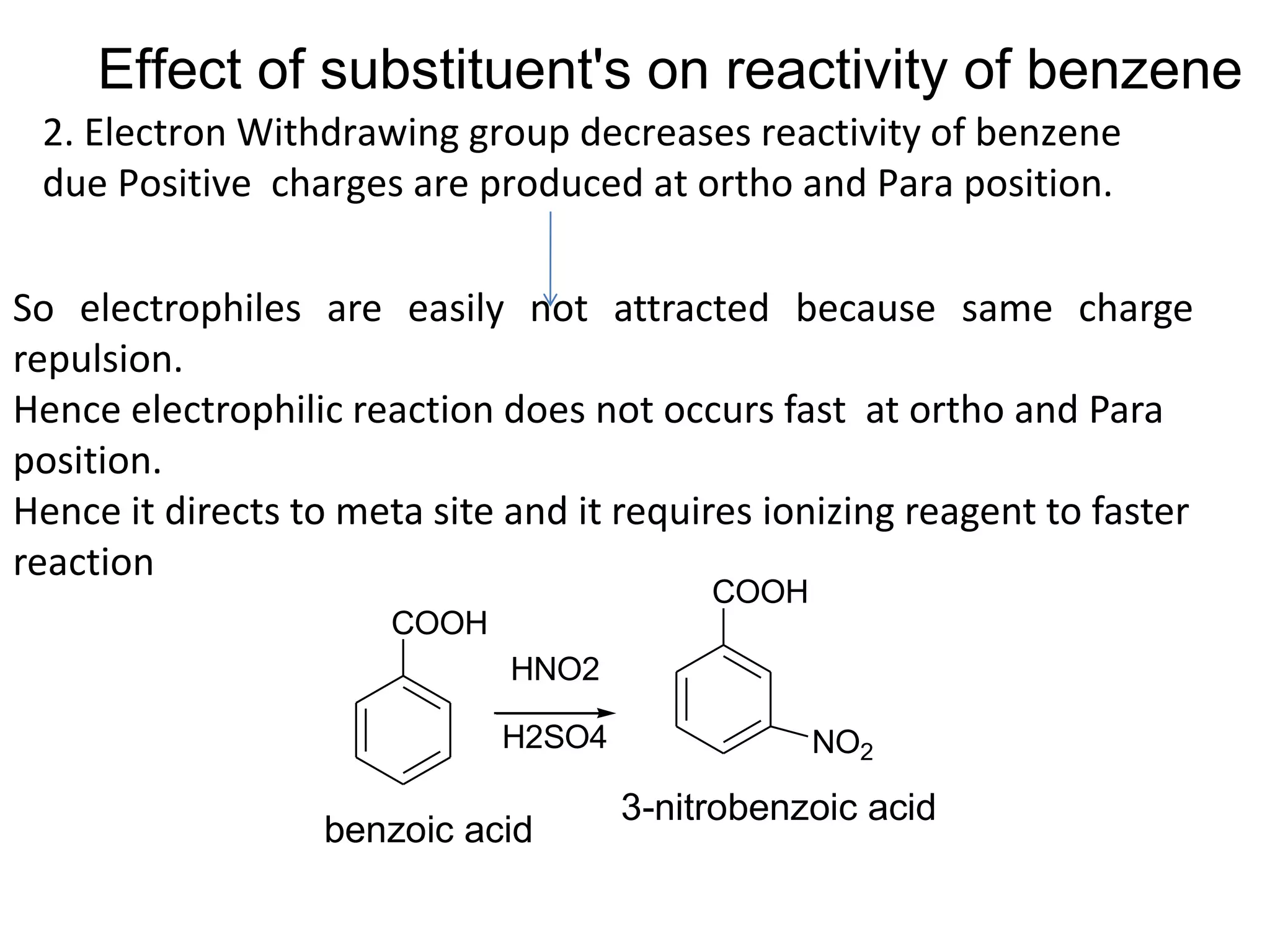
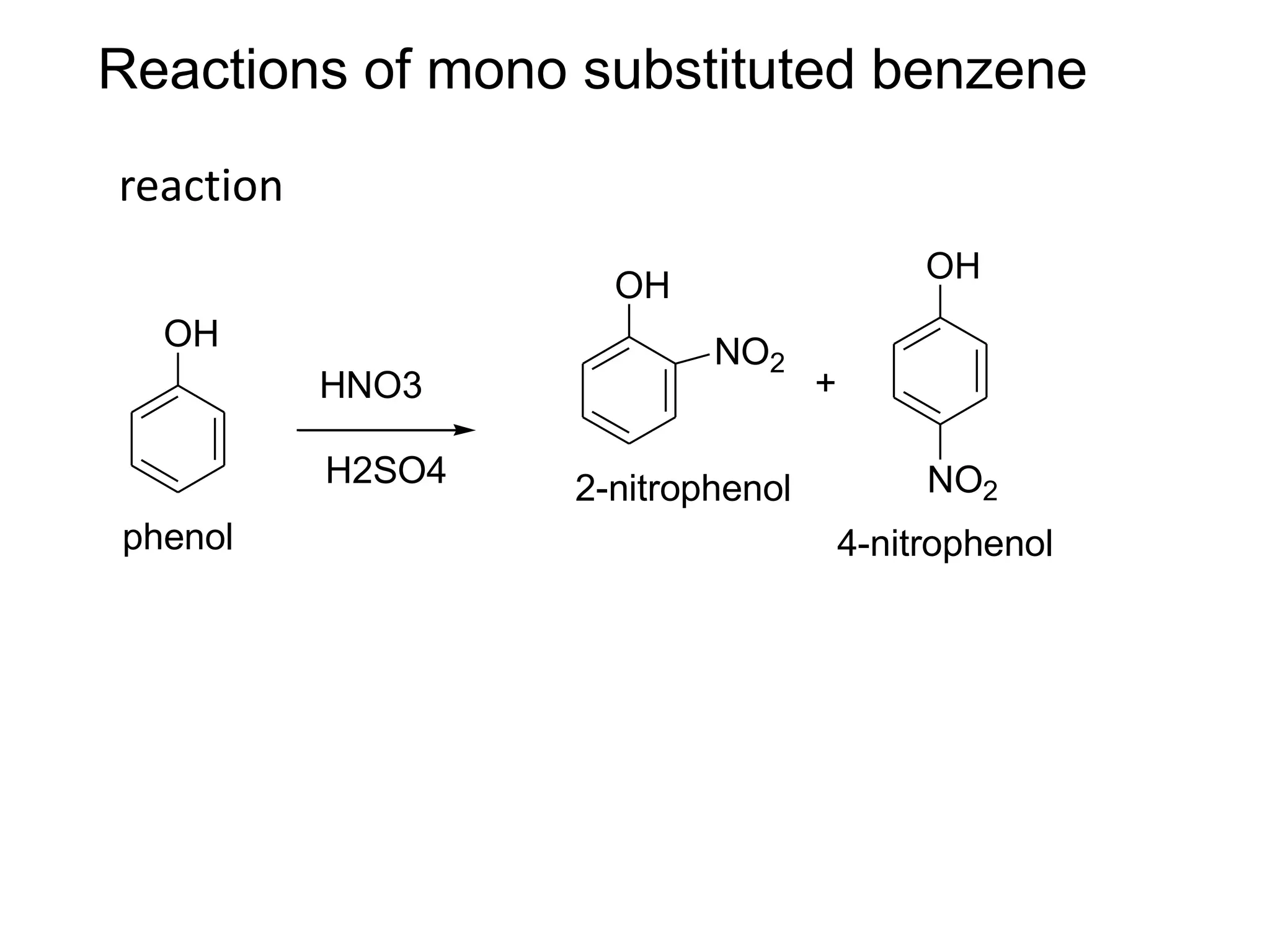
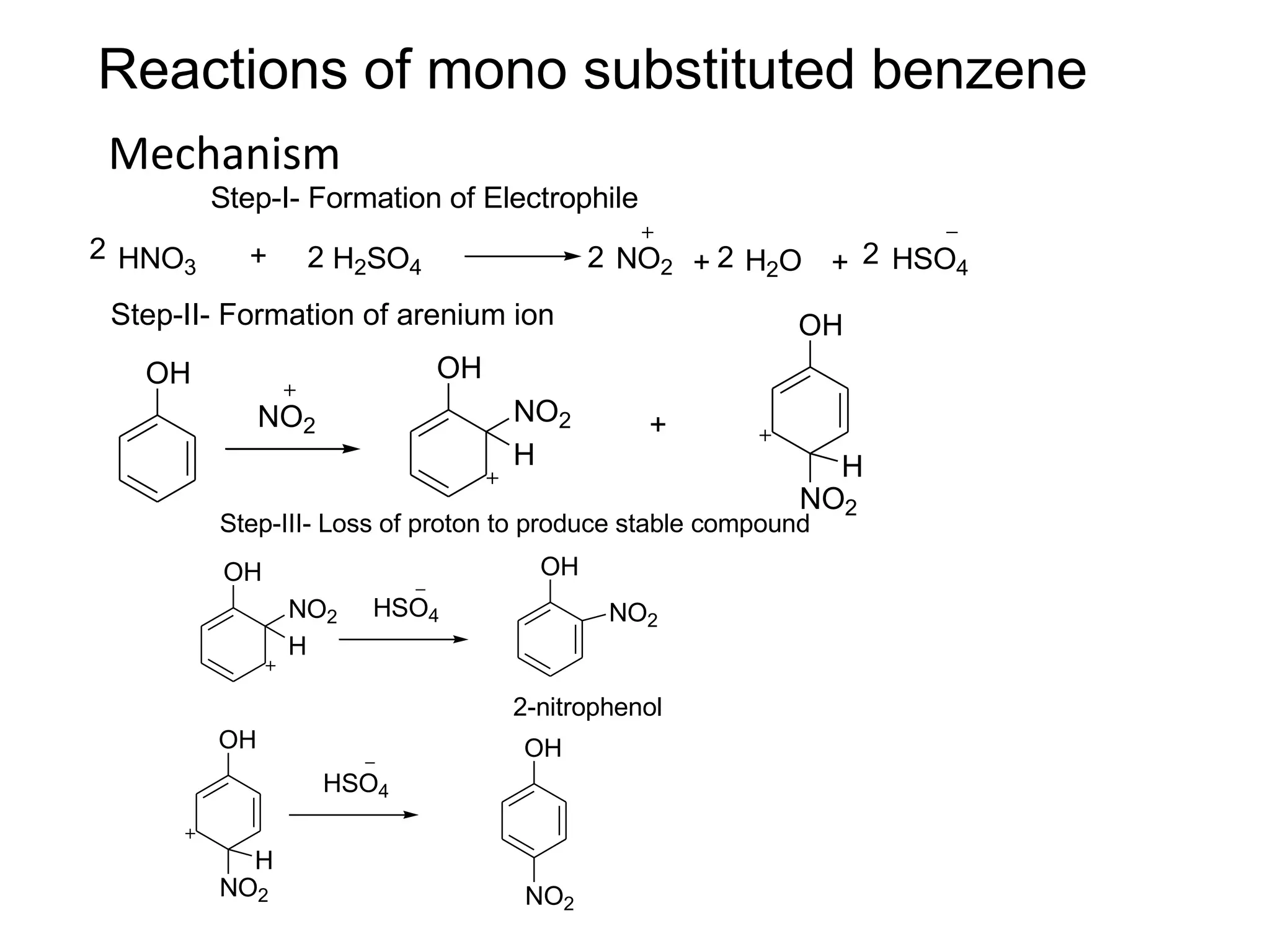
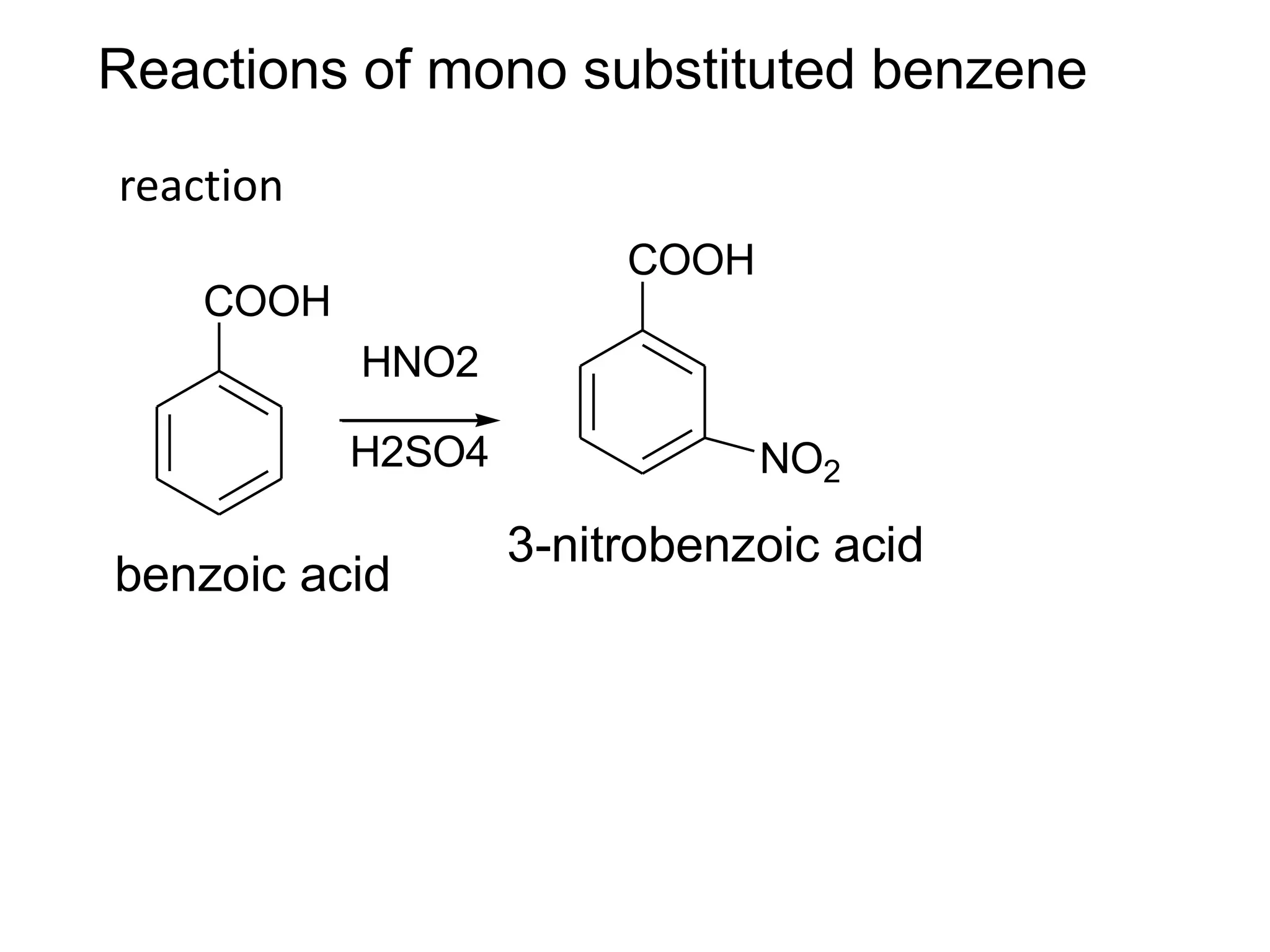
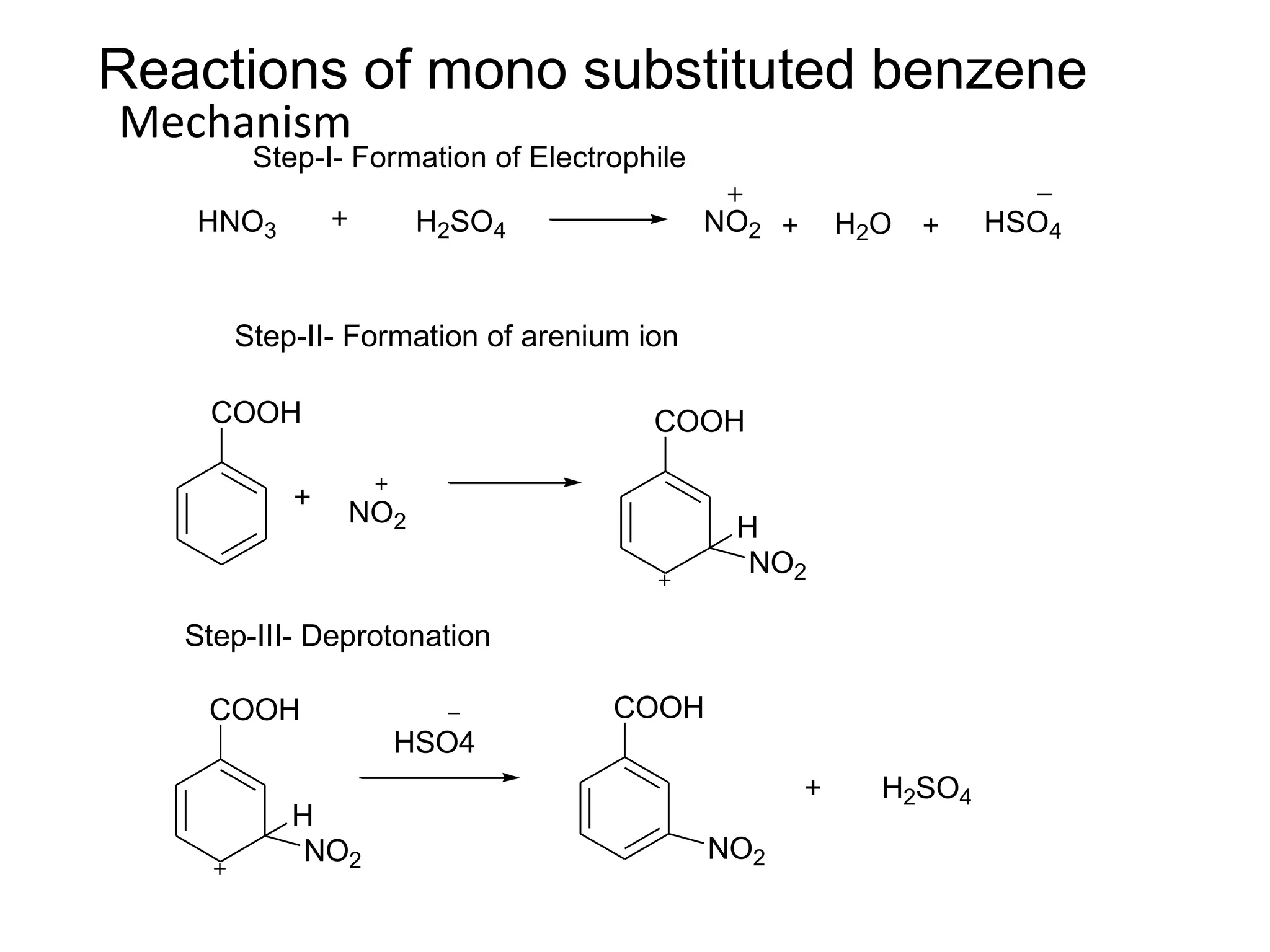
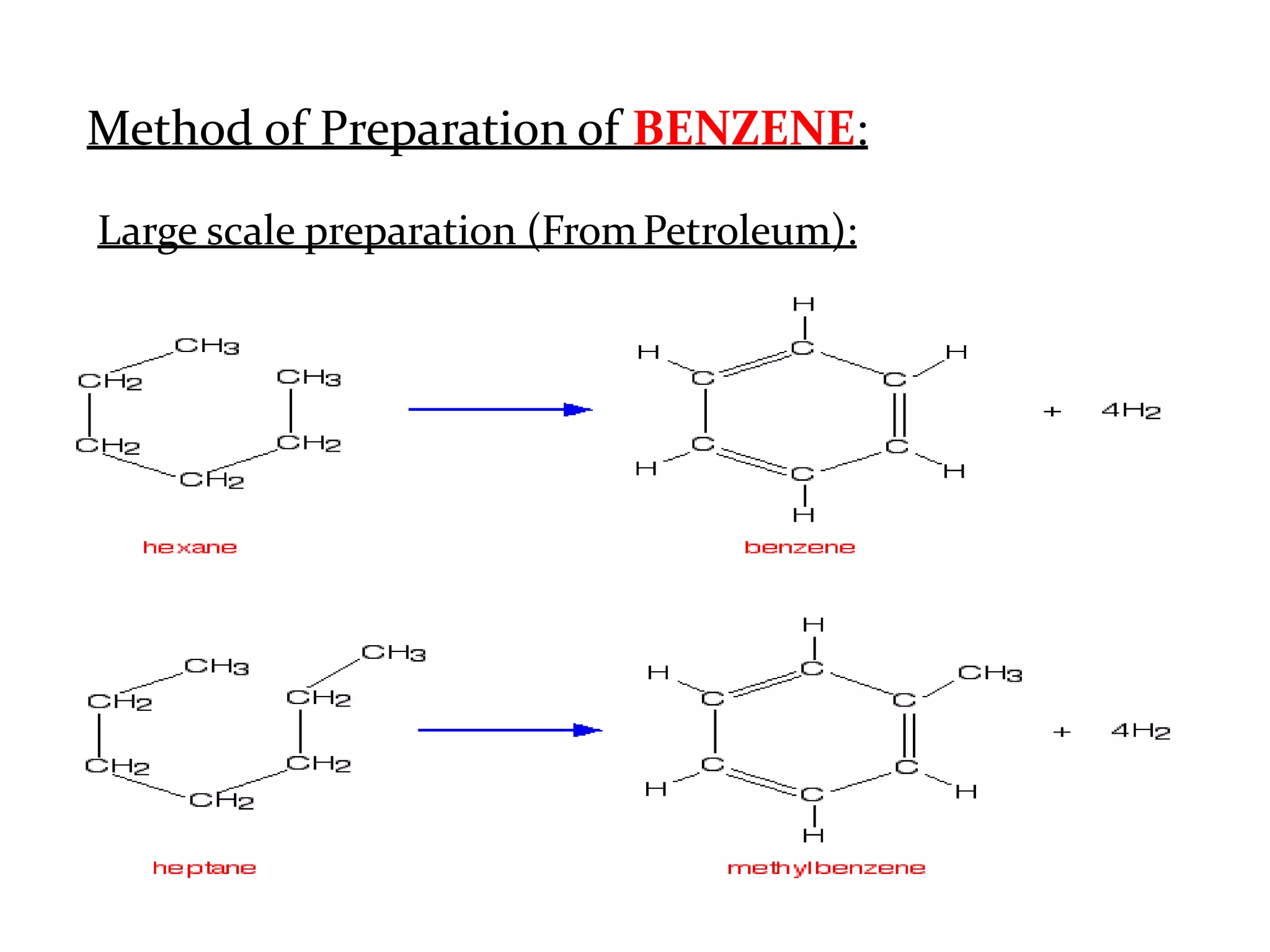
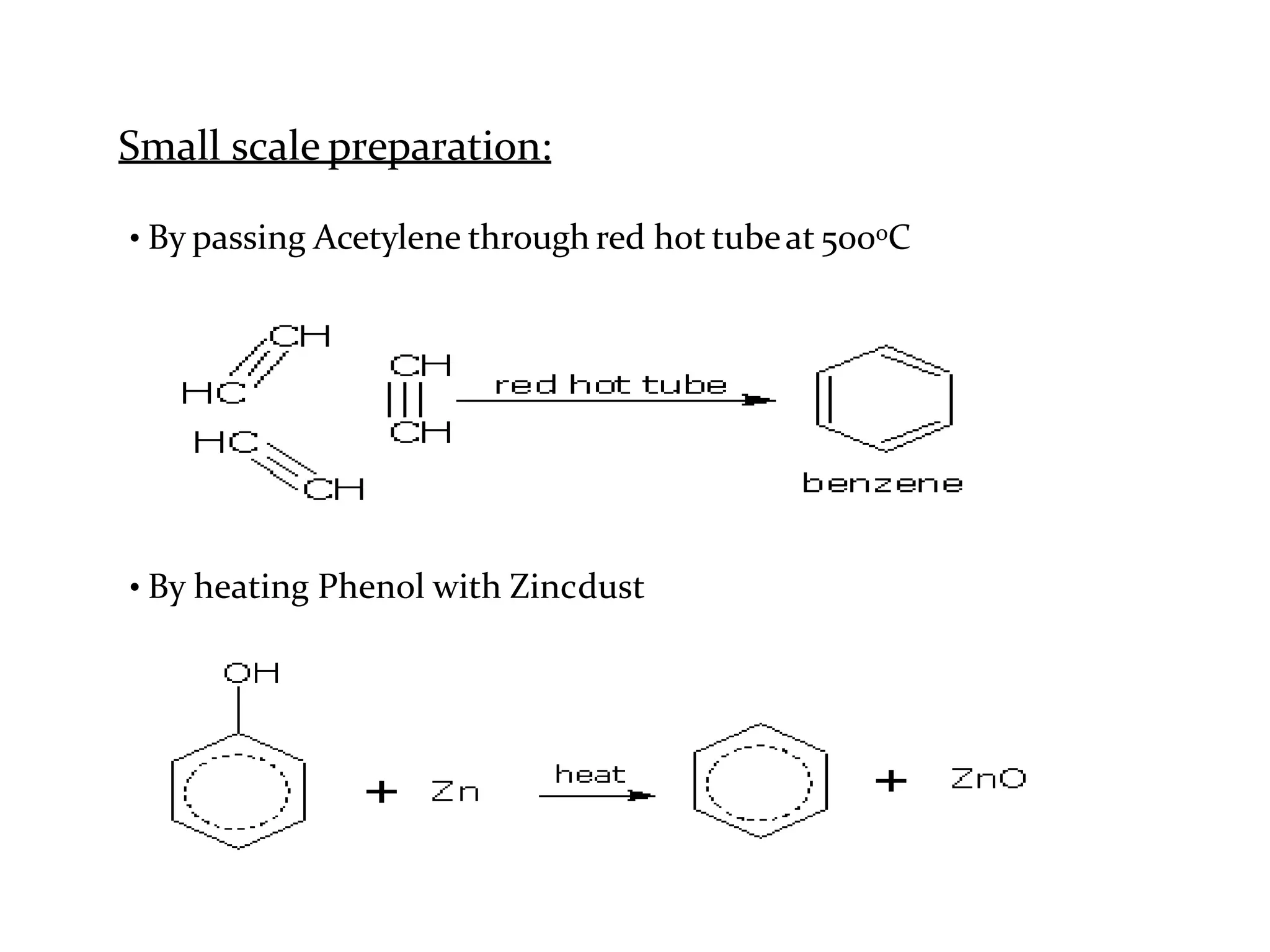
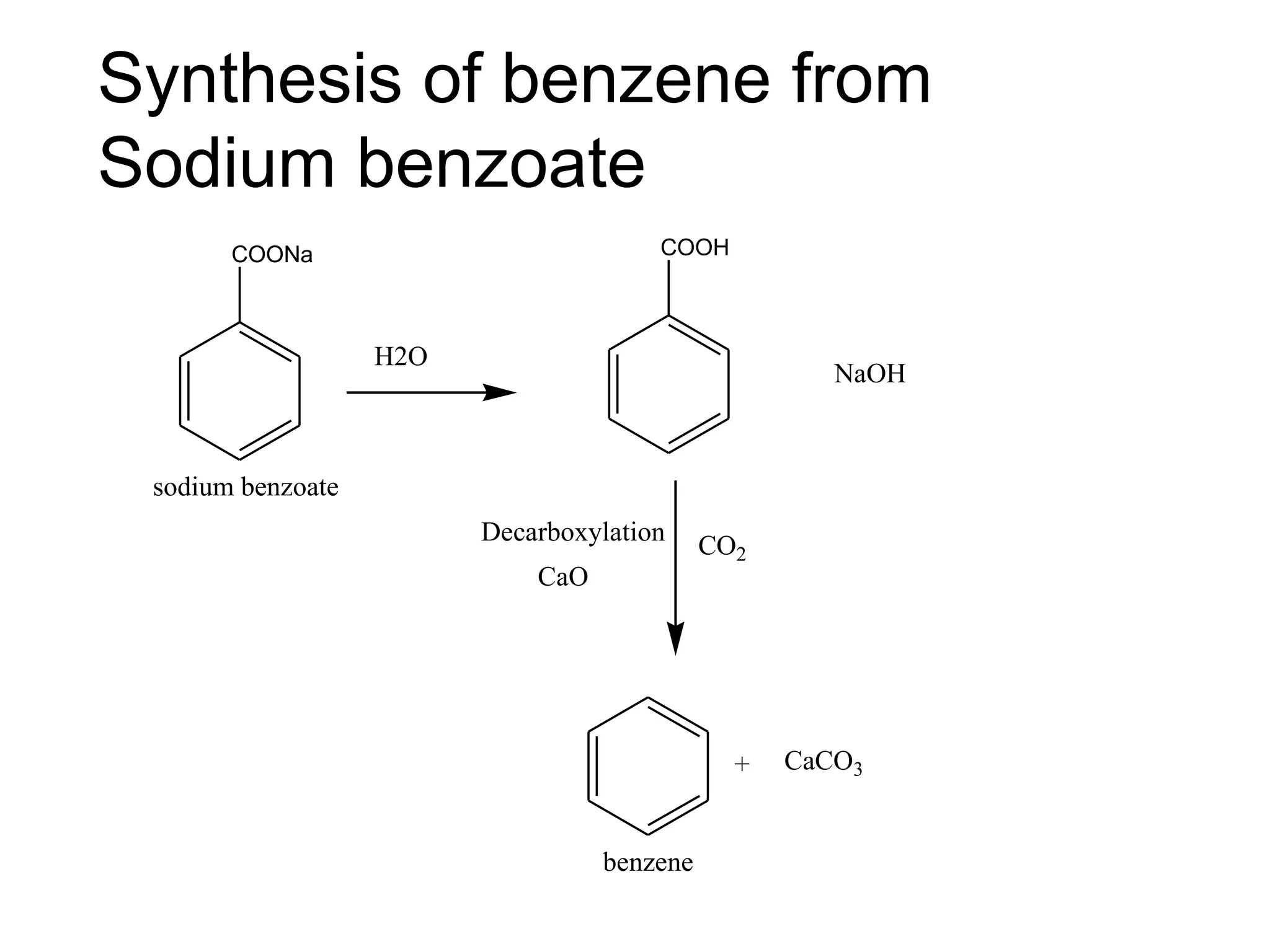
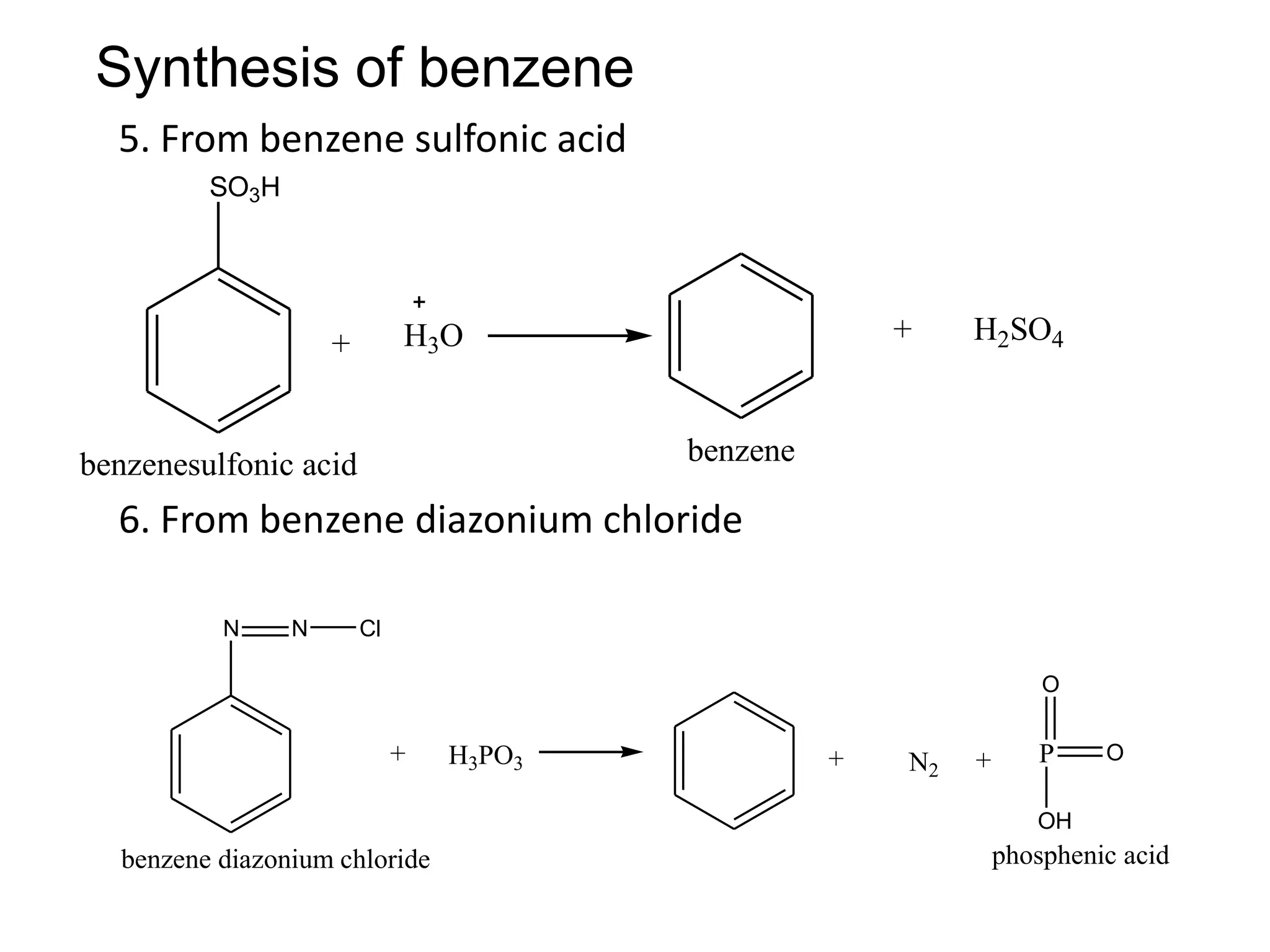
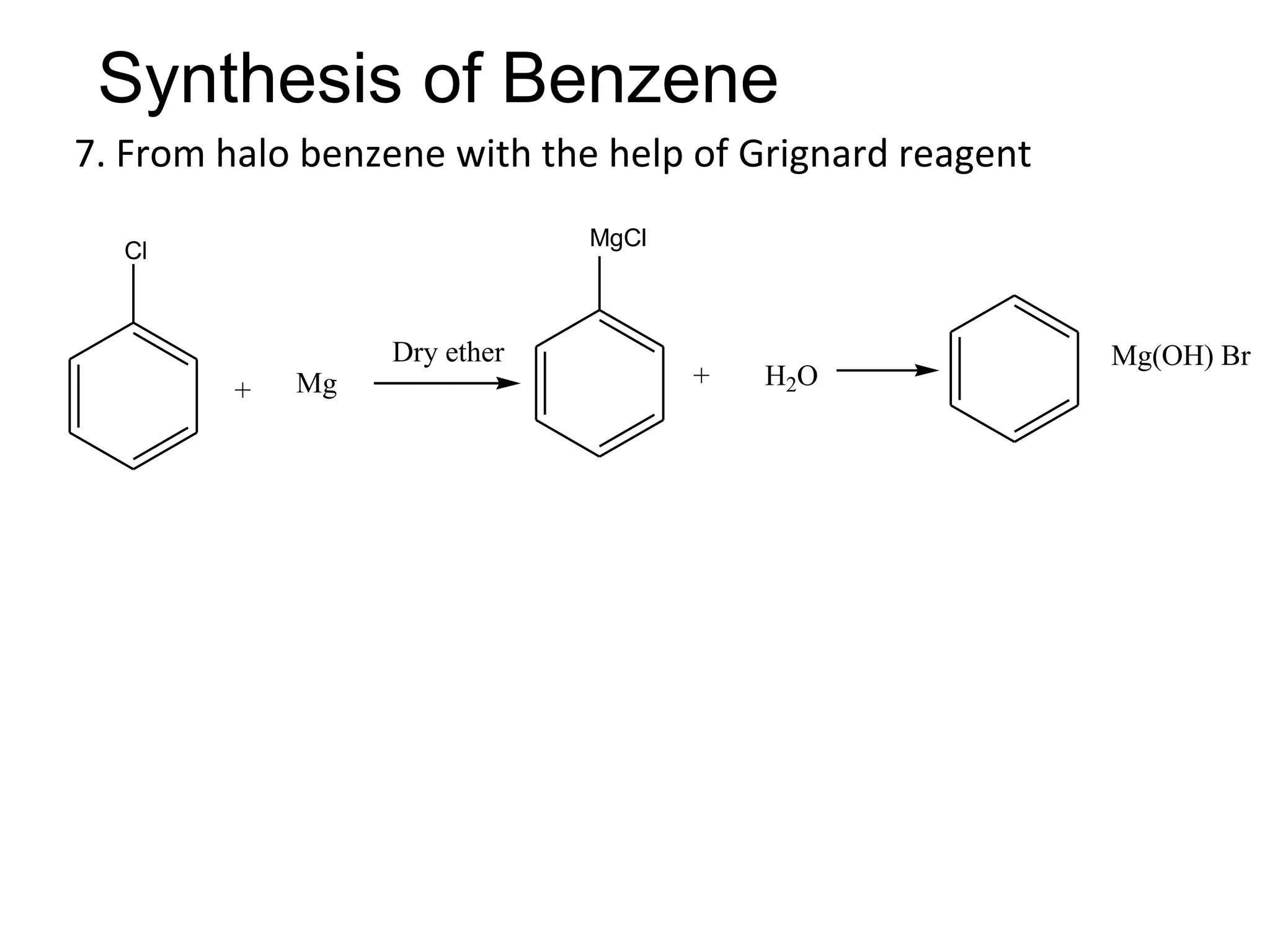
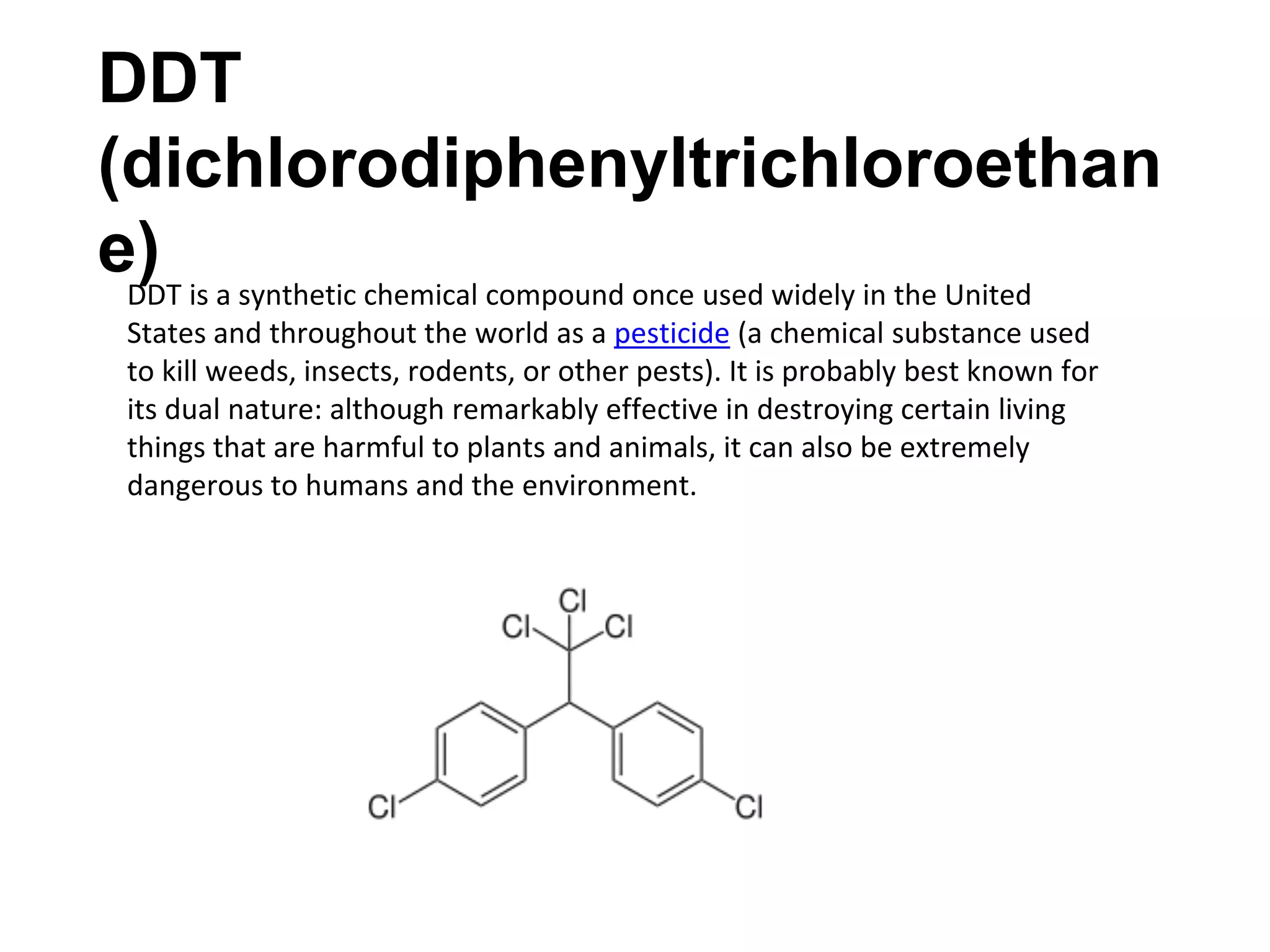
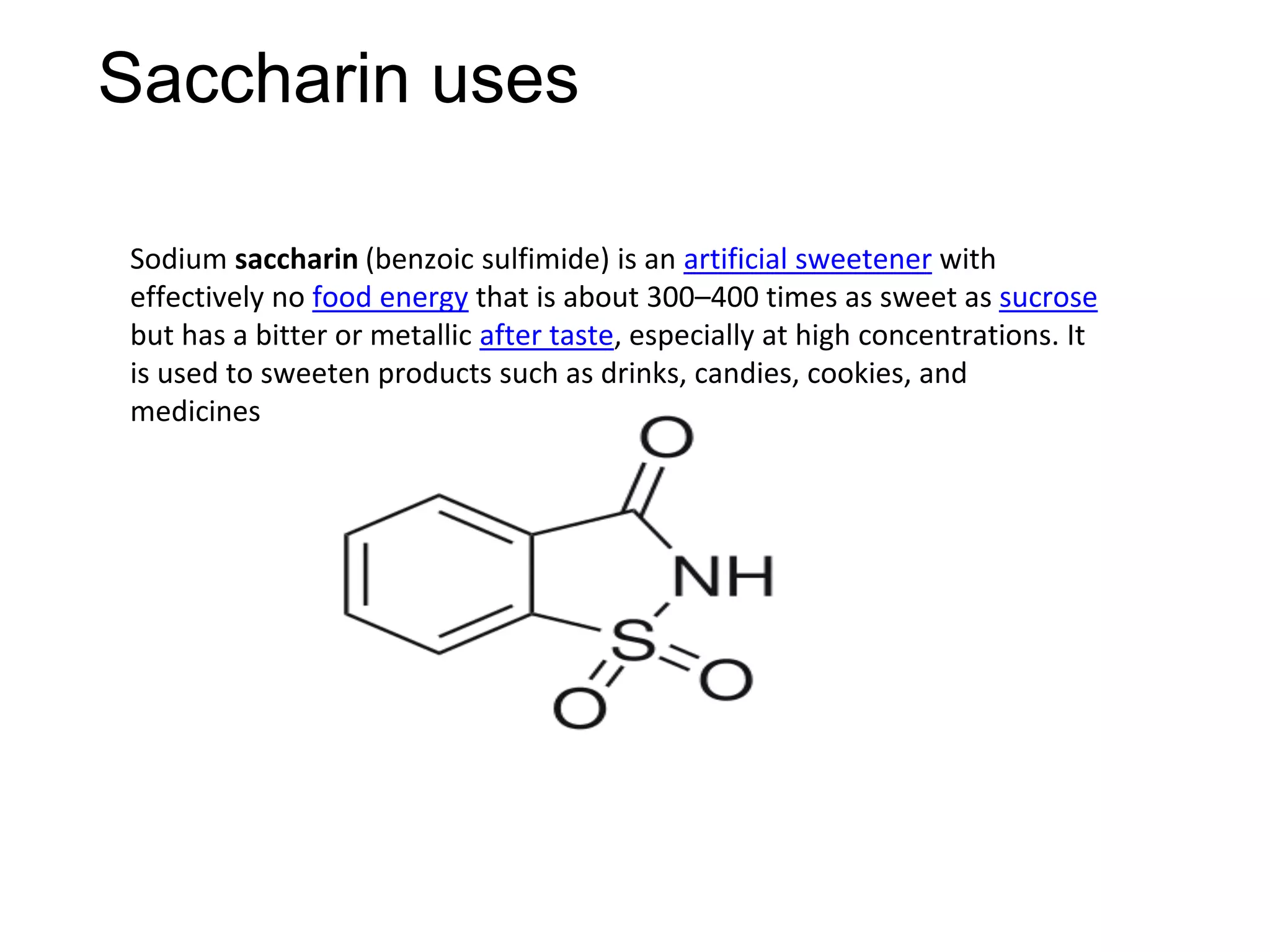
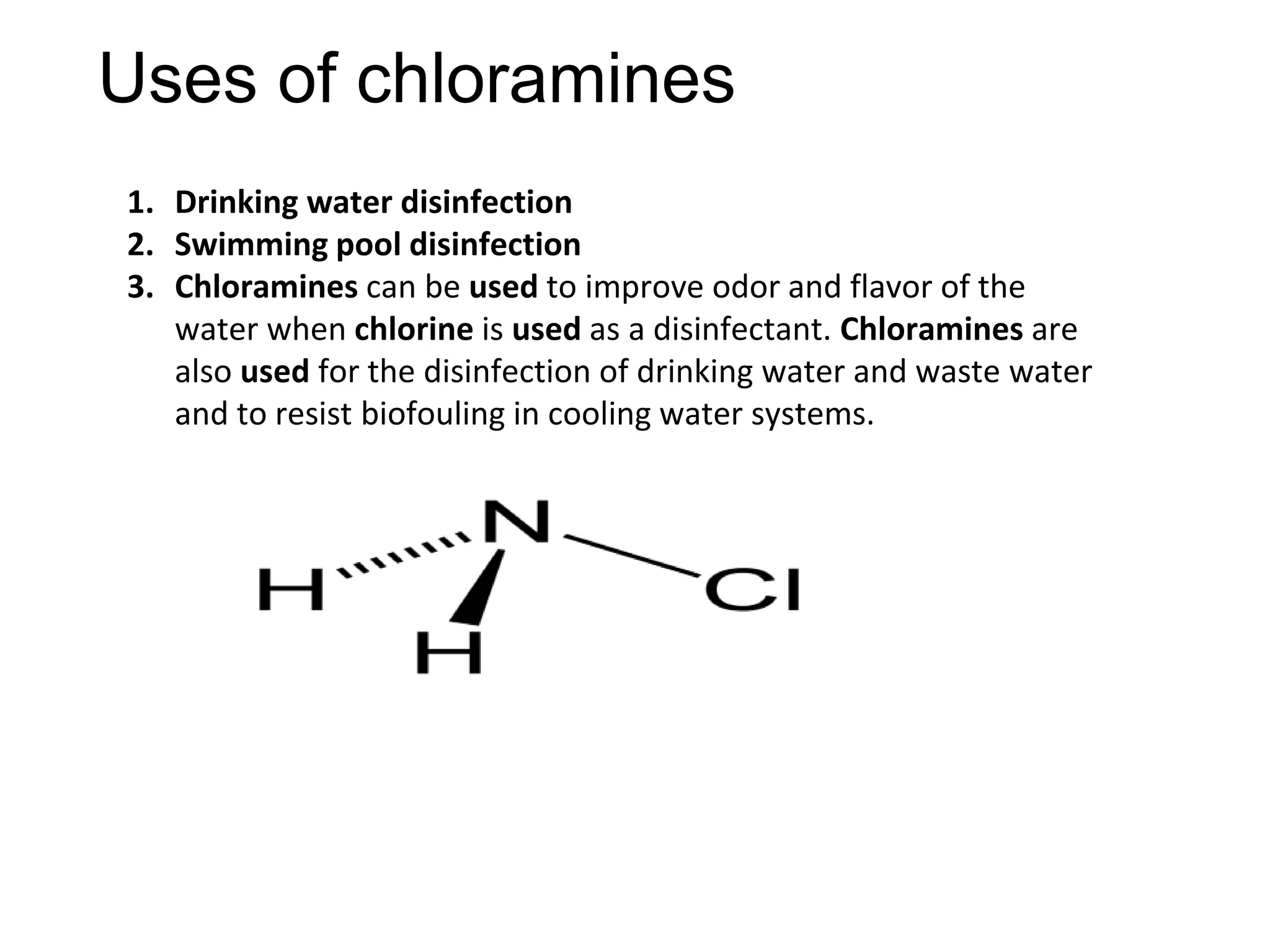
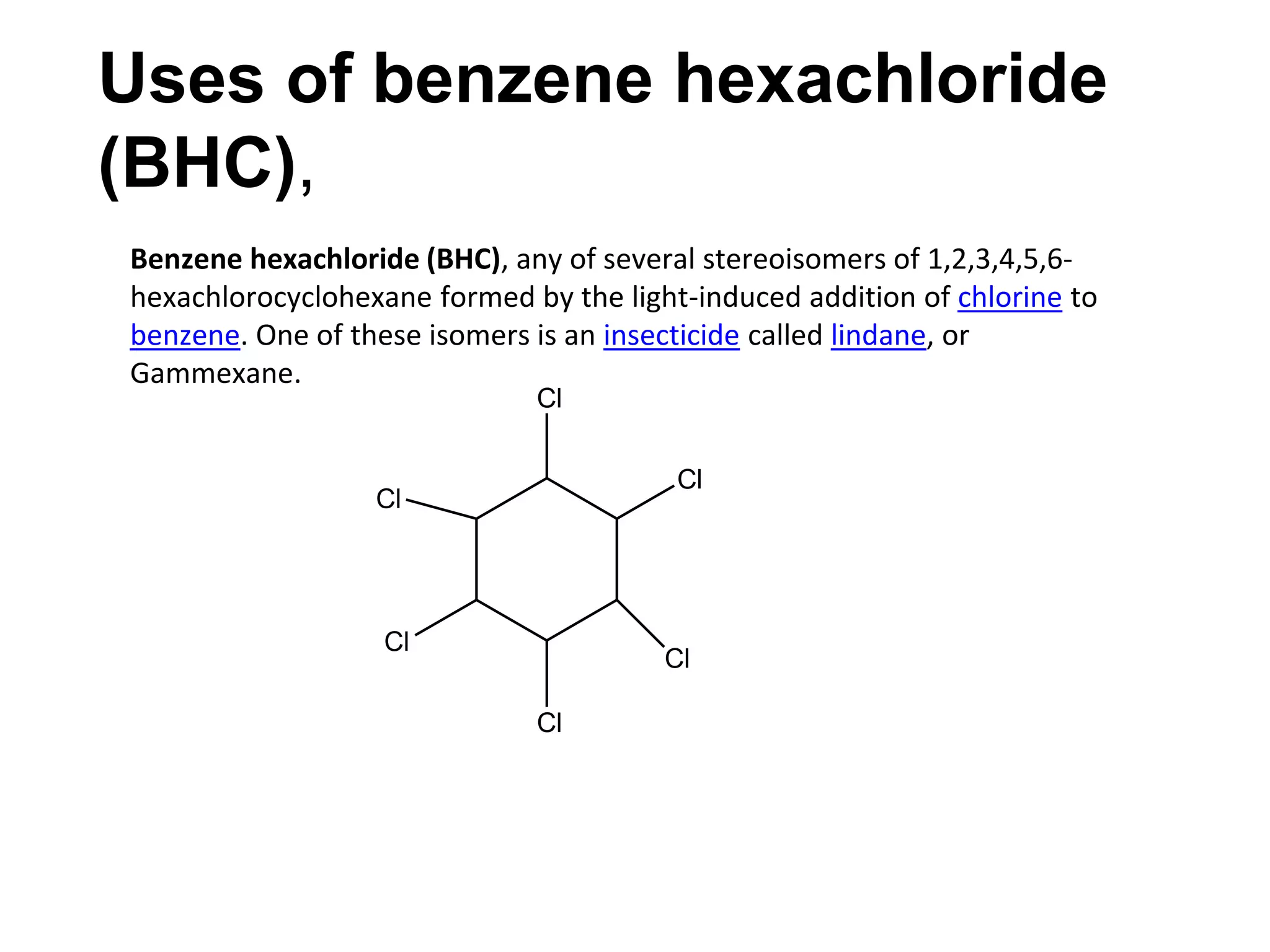
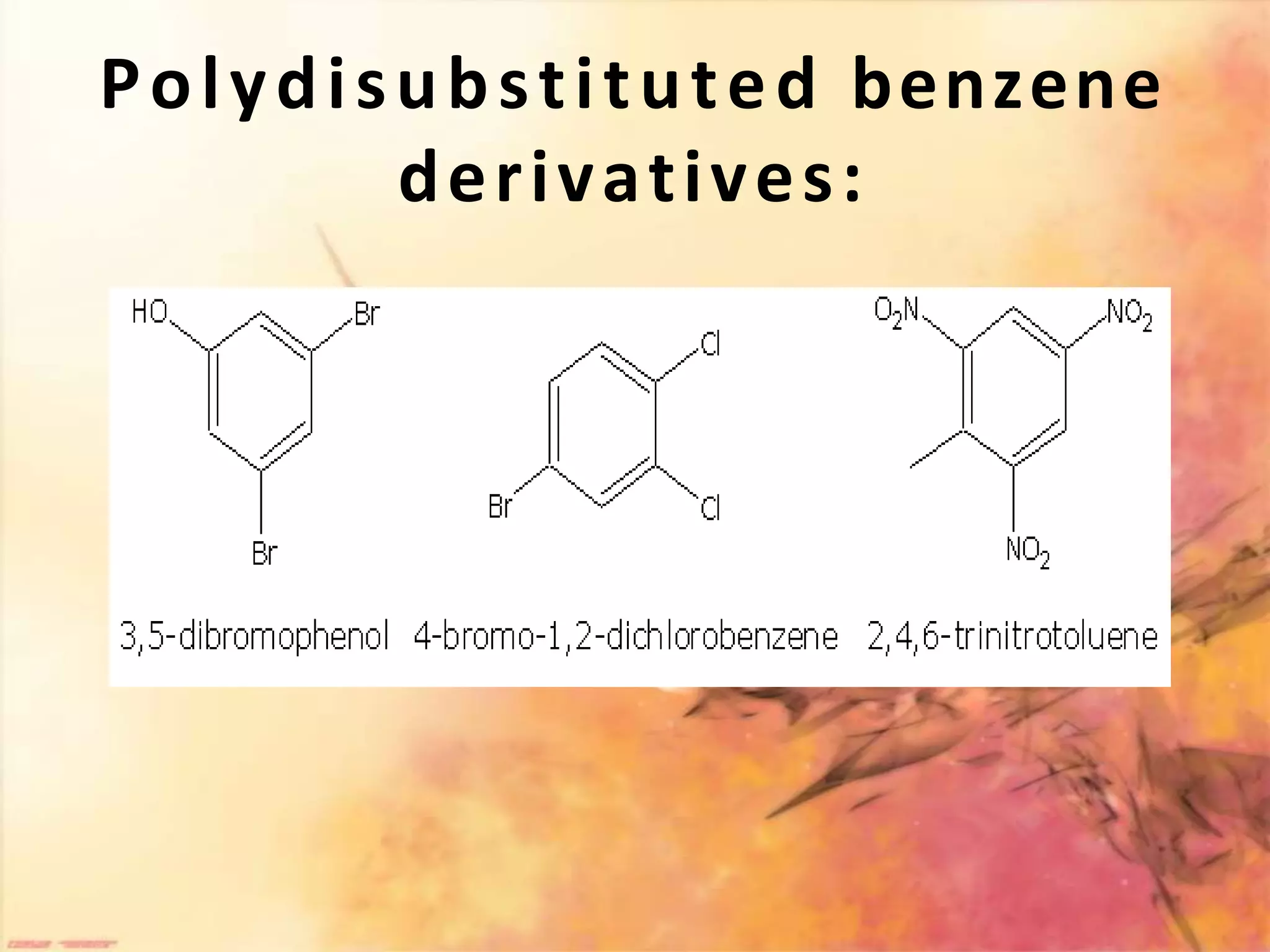
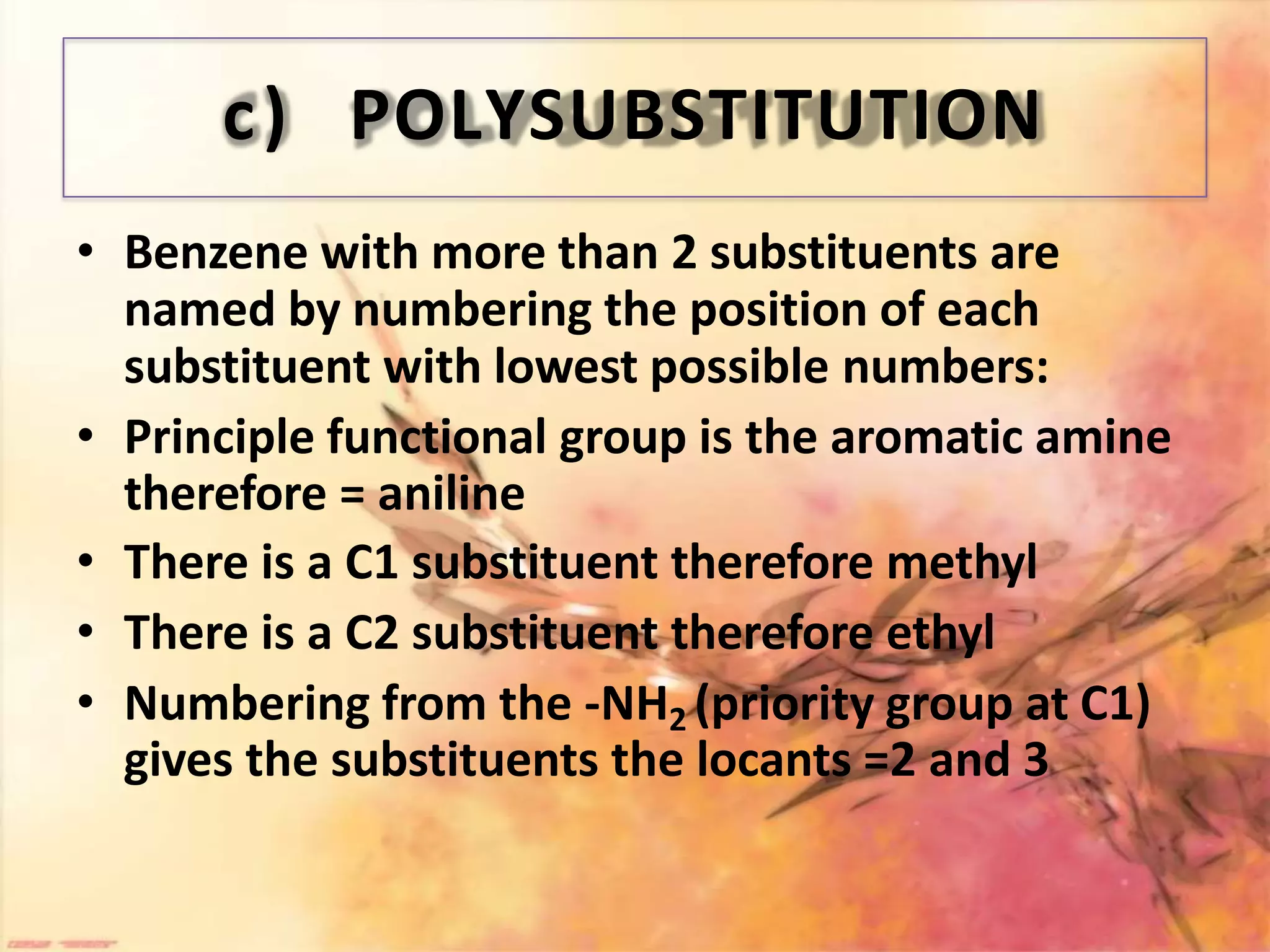
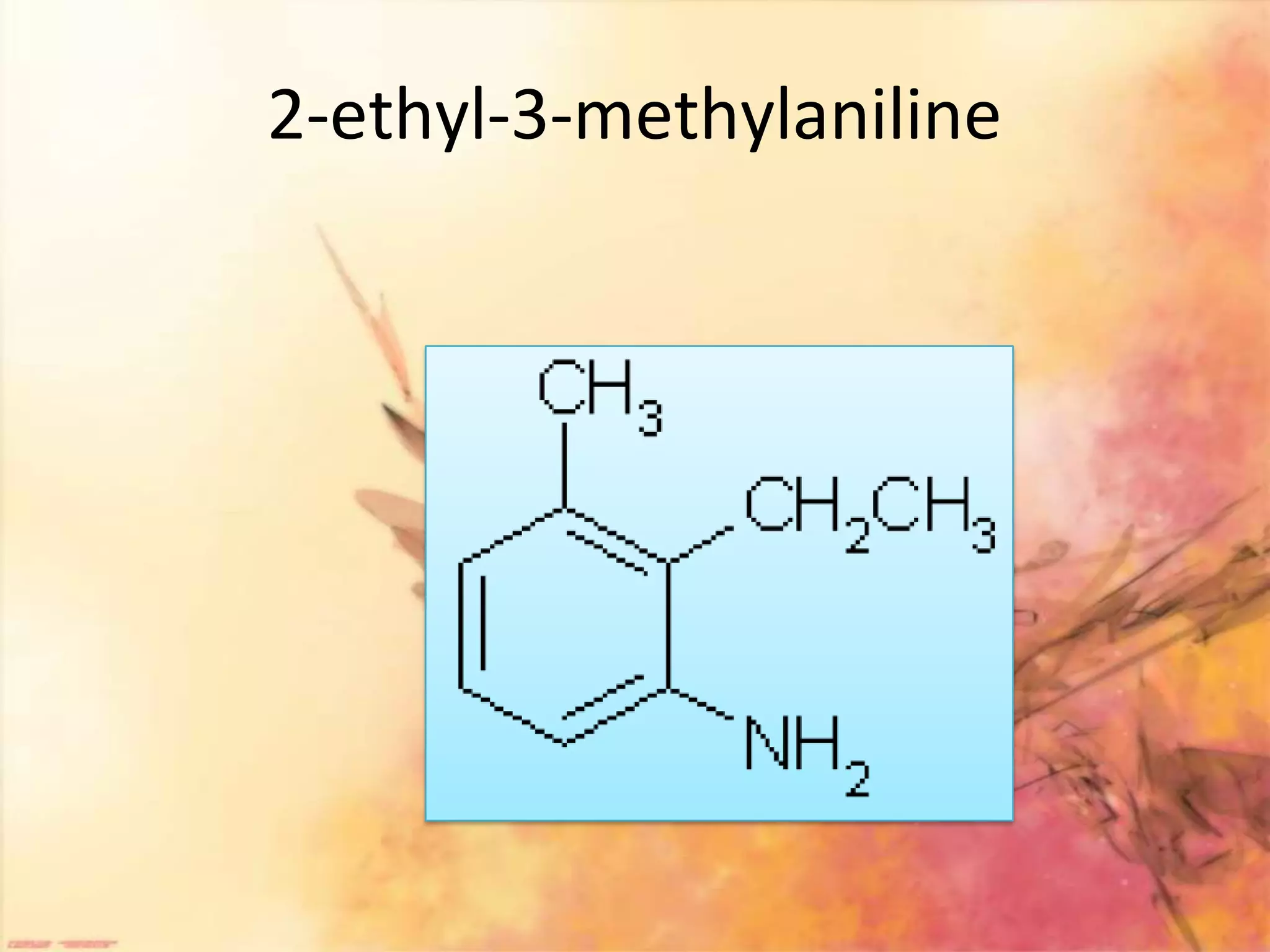
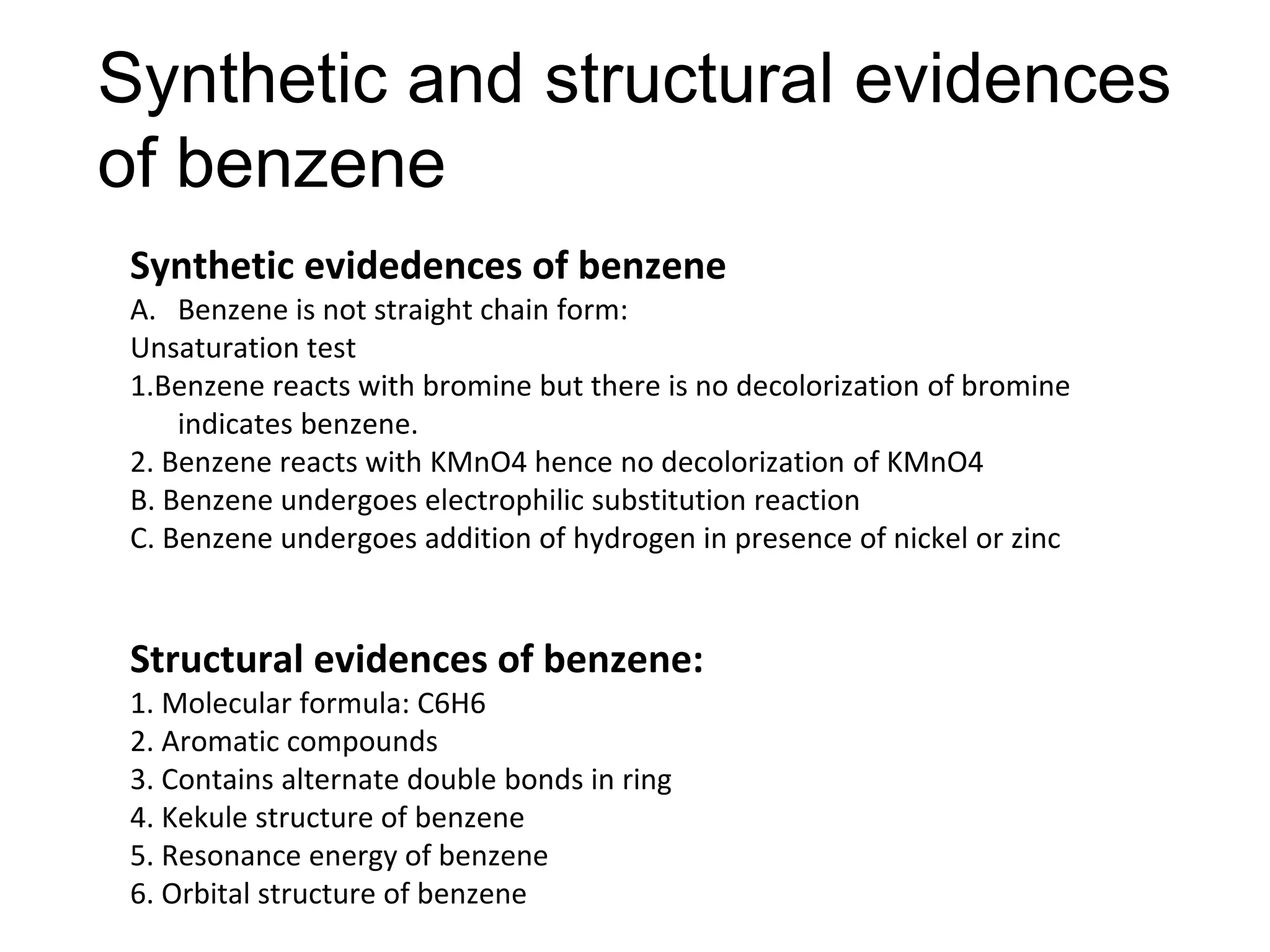
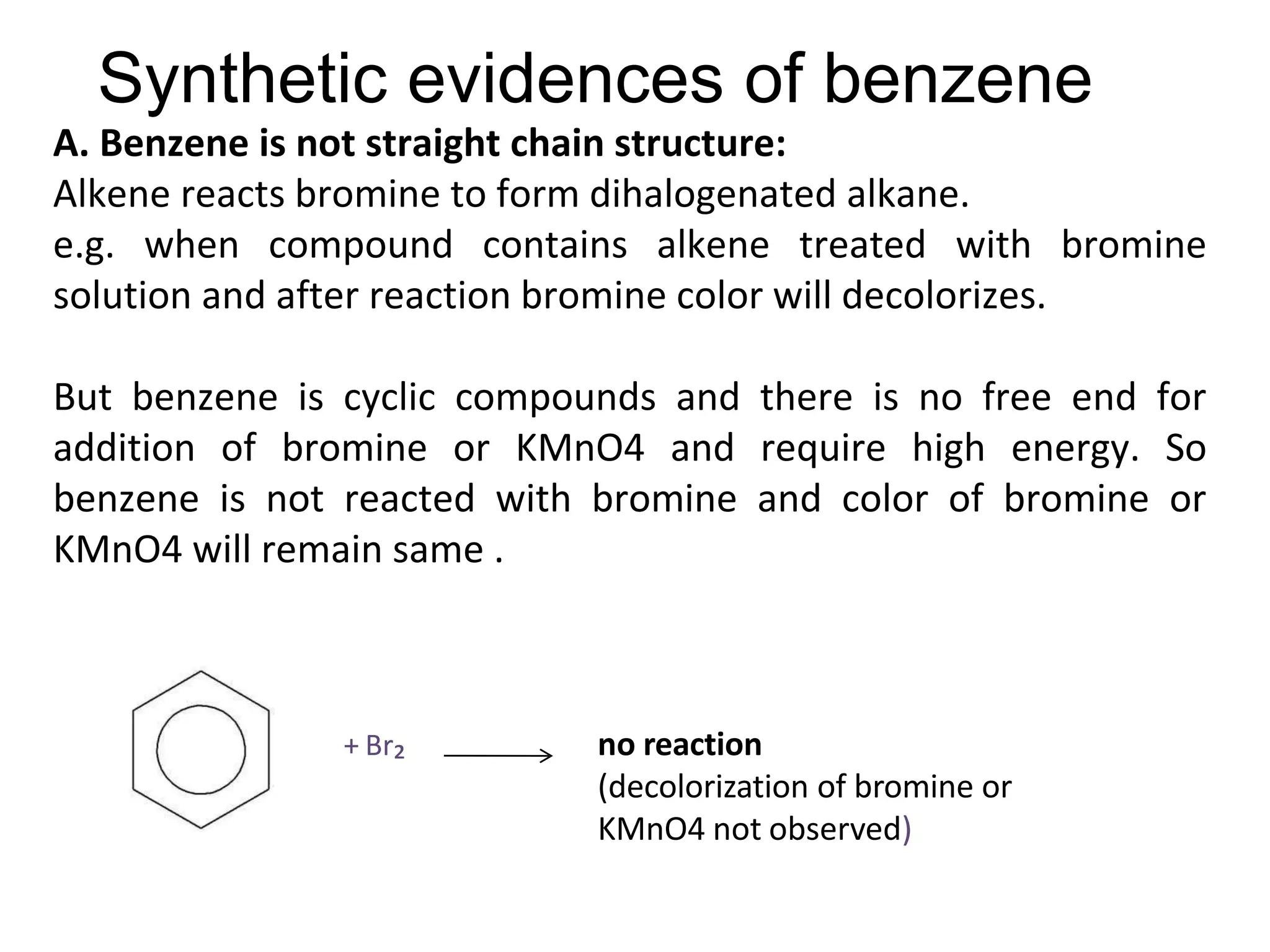
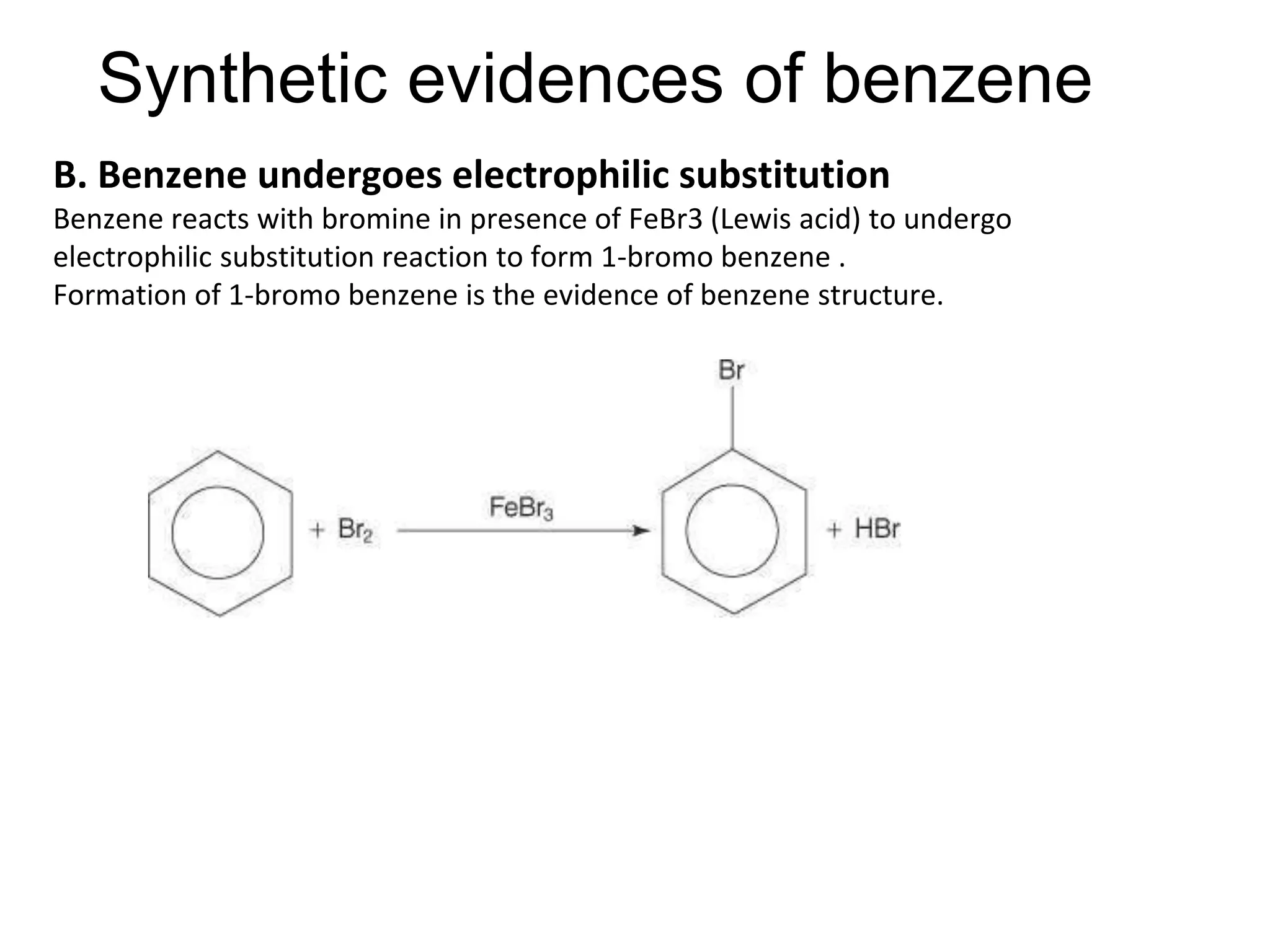
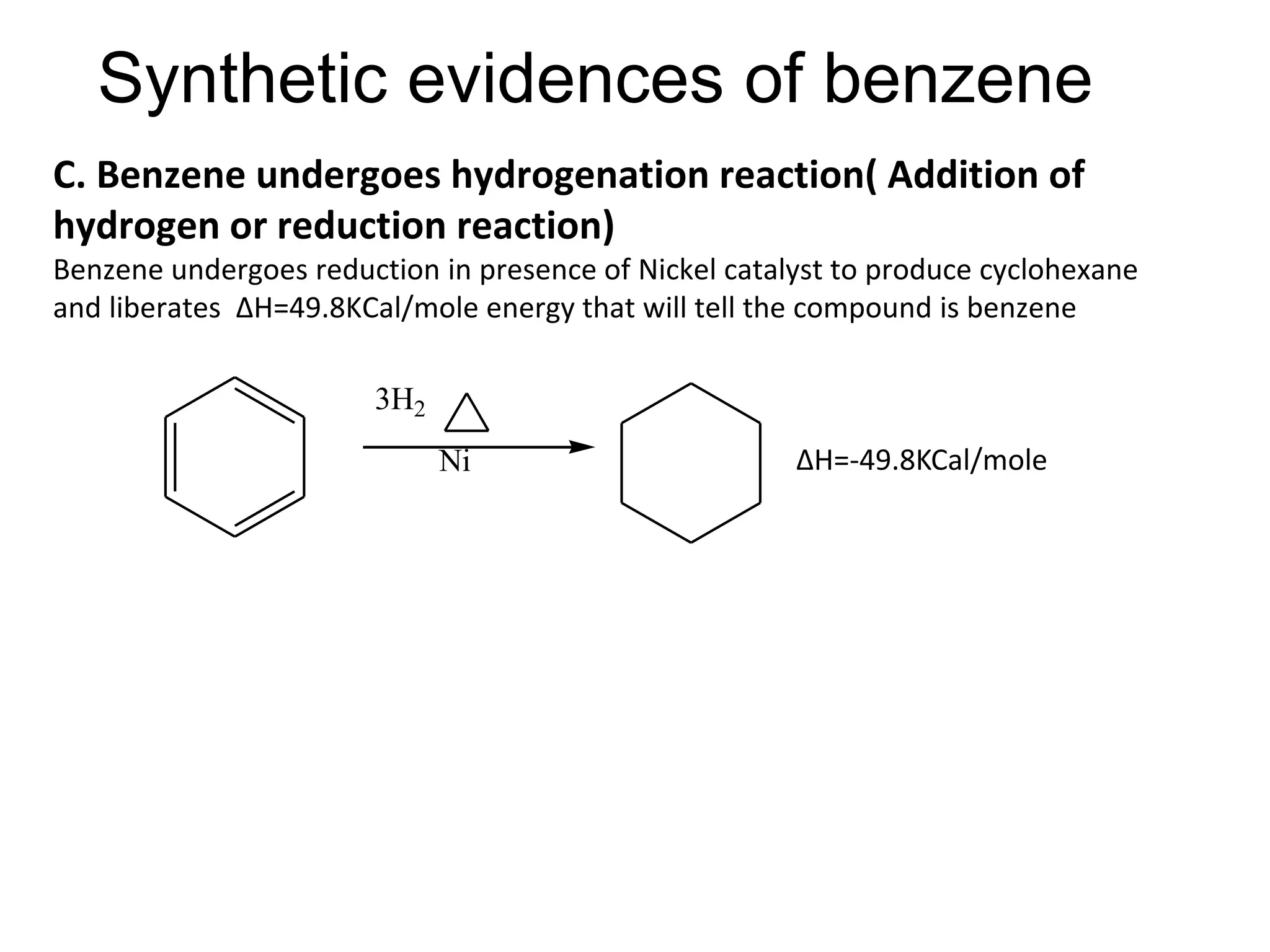
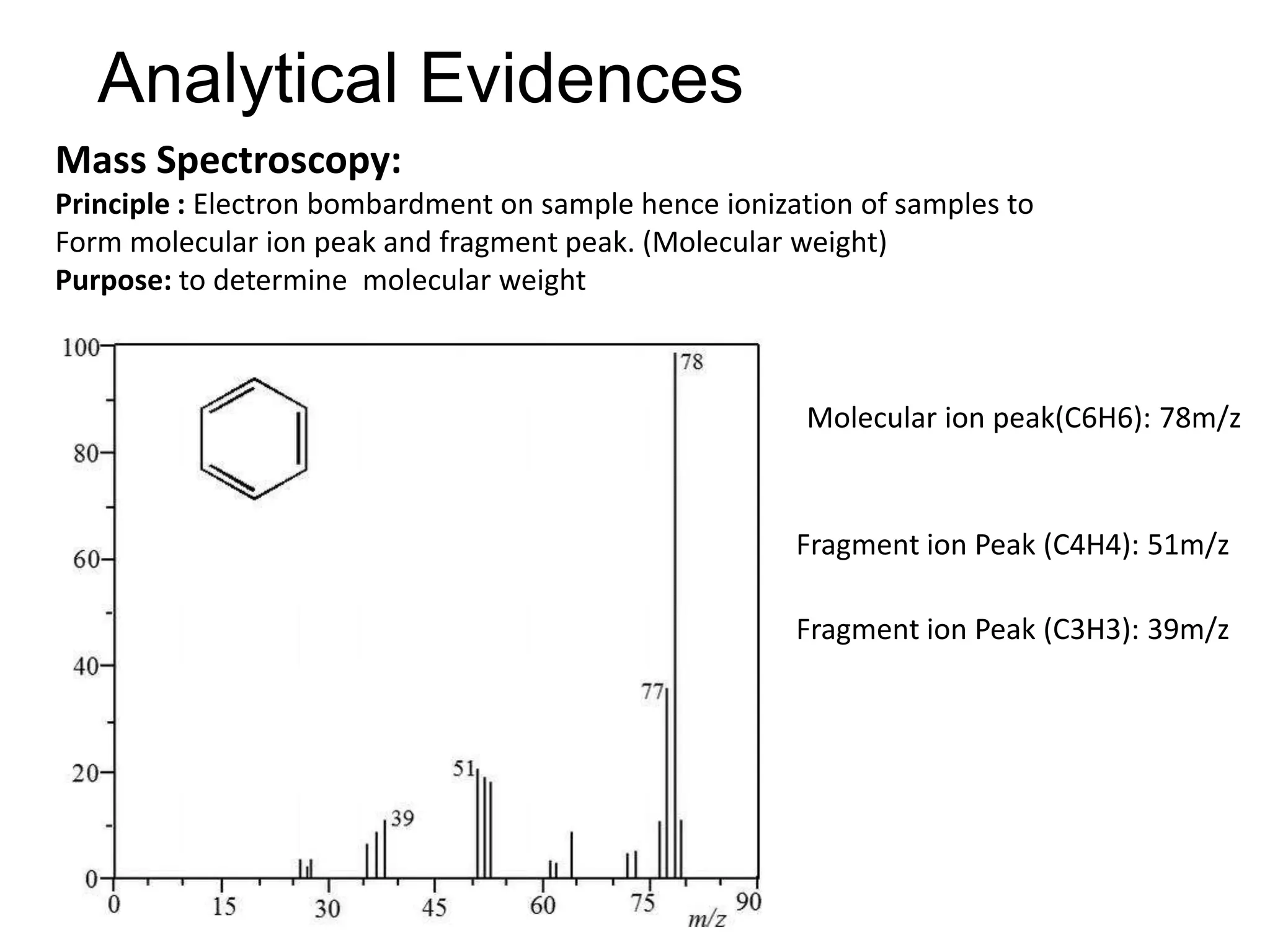
The document provides an extensive review of benzene, including its definition, history, structural characteristics, and chemical properties. It discusses nomenclature, resonance, aromaticity according to Huckel's rule, and various evidences supporting benzene's structure, stability, and reactivity. Additionally, it covers electrophilic substitution reactions and analytical methods such as IR and NMR spectroscopy used to study benzene and related compounds.





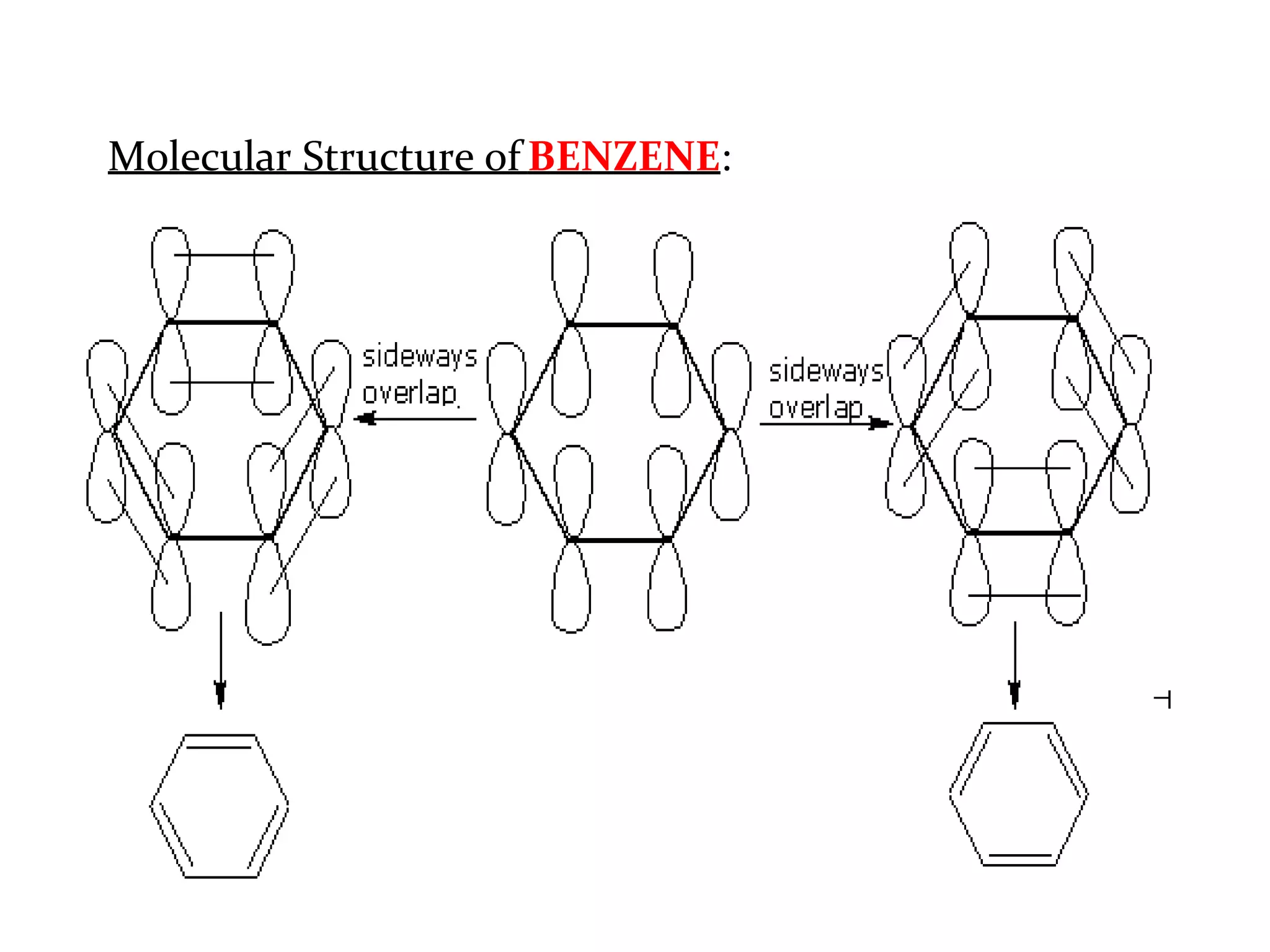


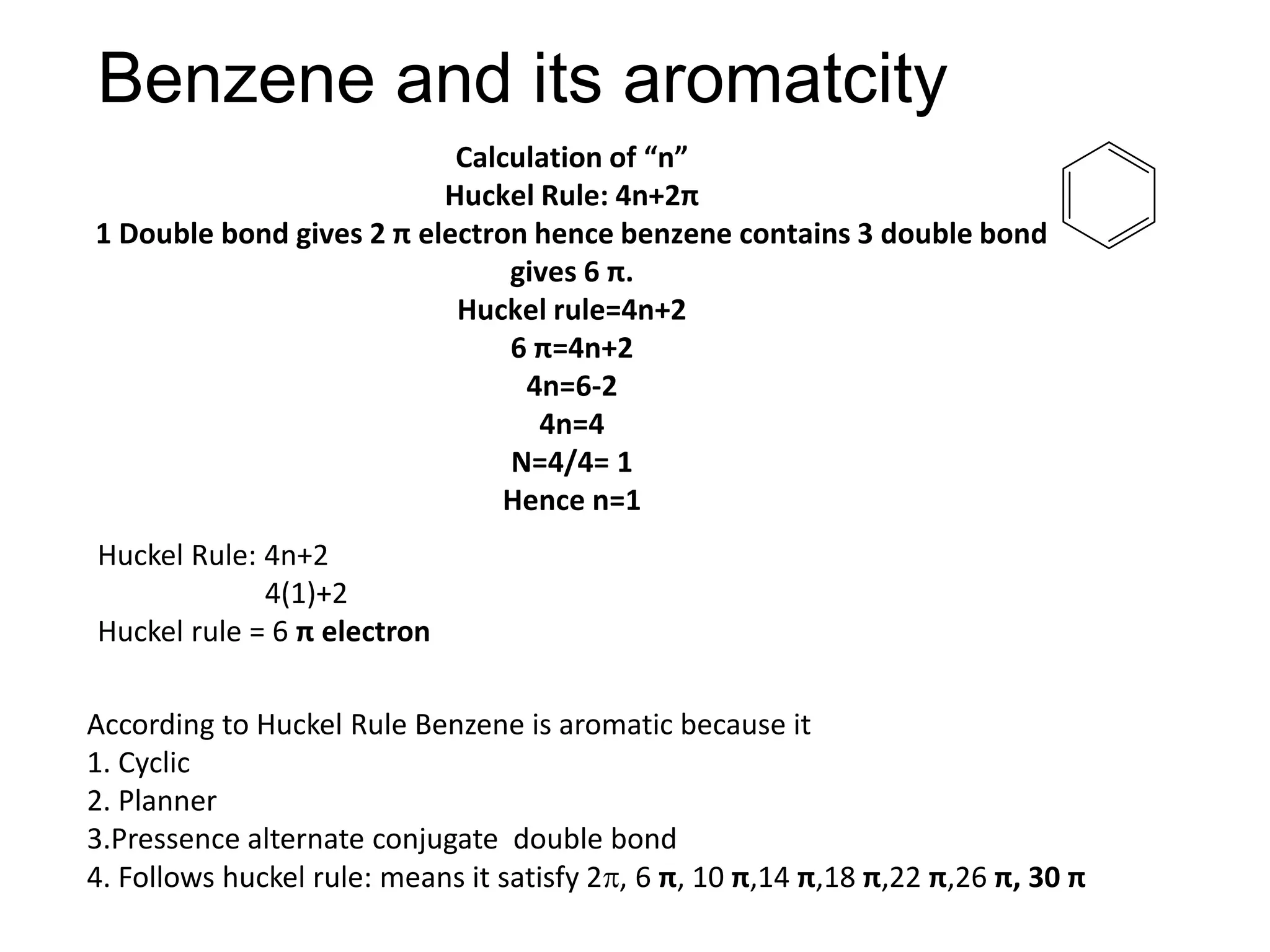
![•Benzene is aromatic and especially stable because it contains 6 electrons.
•Cyclobutadiene is antiaromatic and especially unstable because it contains 4
electrons.
Cont.
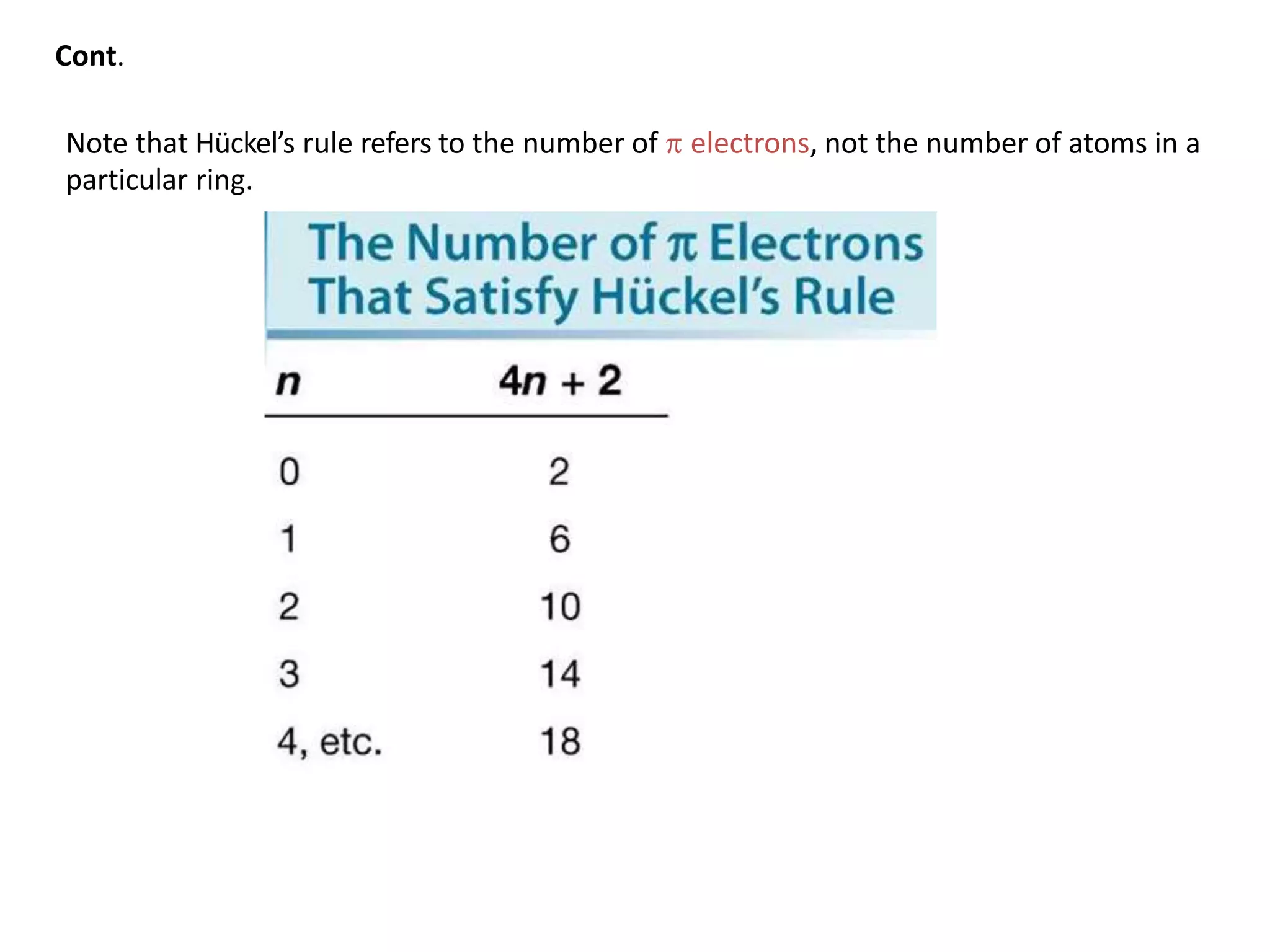
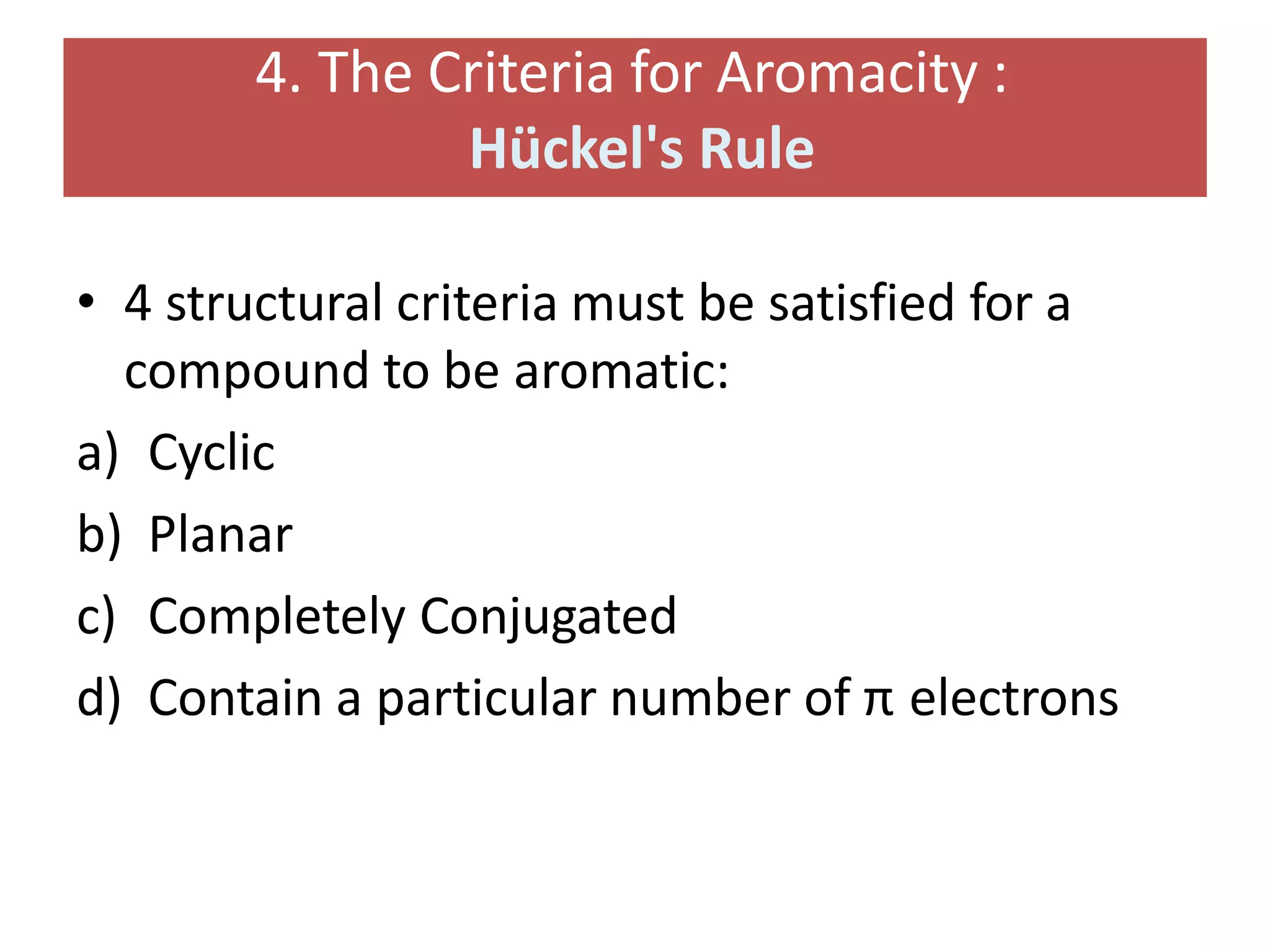
[4] A molecule must satisfy Hückel’s rule, which requires a particular number
of electrons.
Hückel's rule:](https://image.slidesharecdn.com/benzeneanditsderivativesfinal-200725183319/75/Benzene-and-its-derivatives-According-to-PCI-Syllabus-37-2048.jpg)