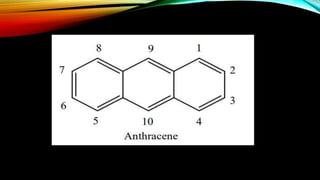
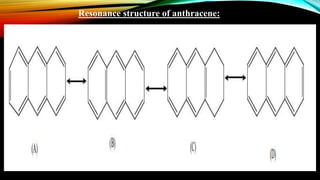
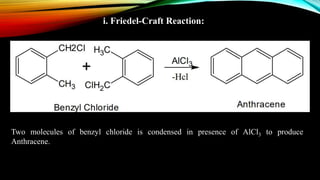
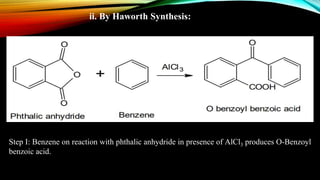
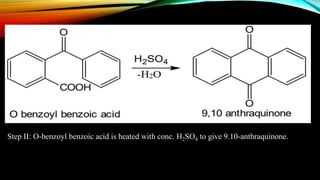
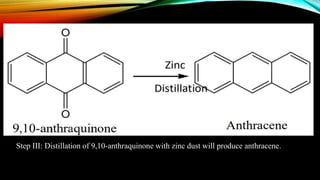
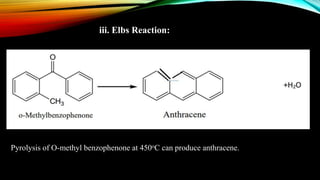
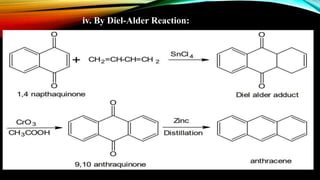
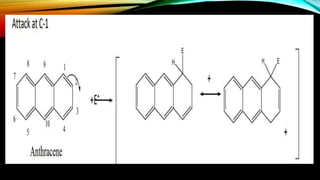
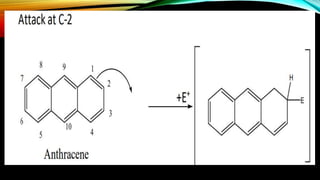
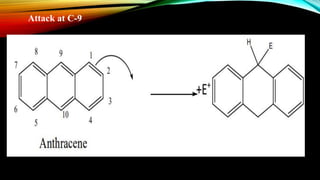
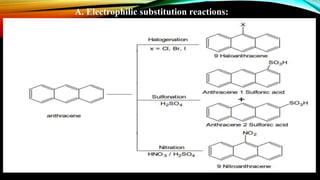
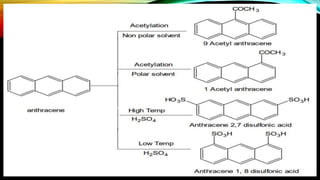
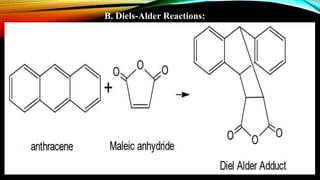
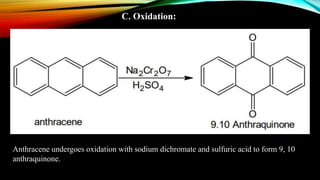
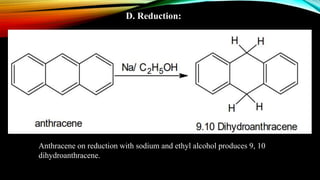
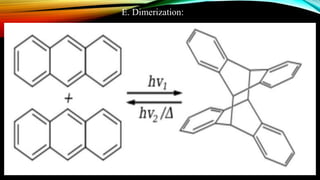
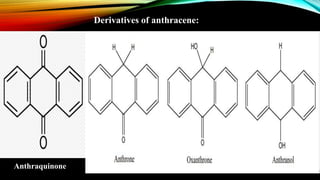
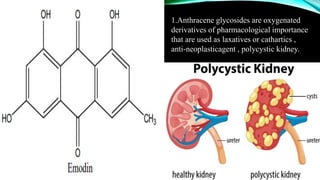
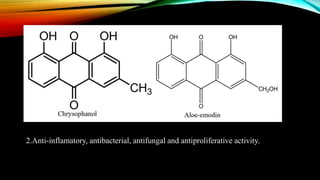
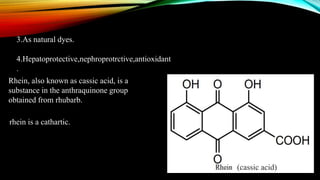
Anthracene is a colorless polycyclic aromatic hydrocarbon present in coal tar. It is made up of three fused benzene rings. Resonance structures show that the C1-C2 bond is shorter than the C2-C3 bond due to double bond character. Anthracene is less aromatic than benzene and behaves more like an unsaturated hydrocarbon. It undergoes addition and electrophilic substitution reactions preferentially at the C9 and C10 positions. Anthracene derivatives have pharmacological uses as laxatives, anticancer agents, and treatments for kidney diseases.