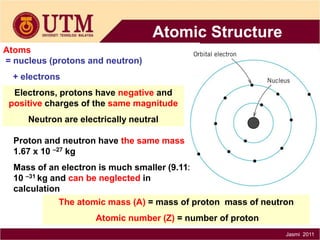
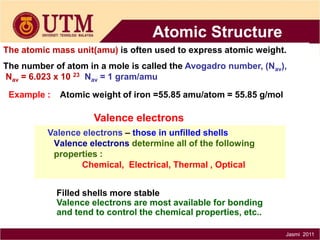
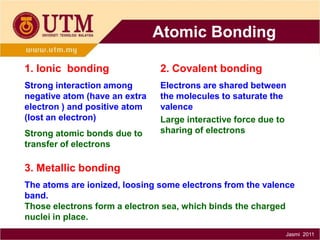
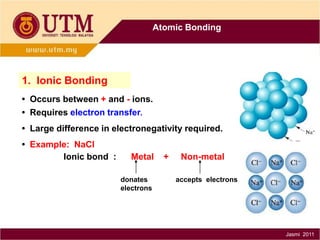
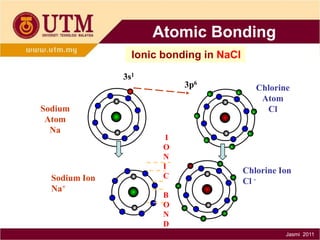
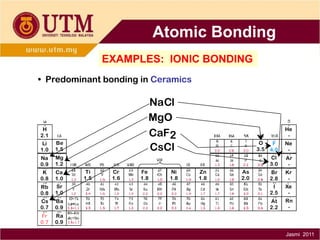
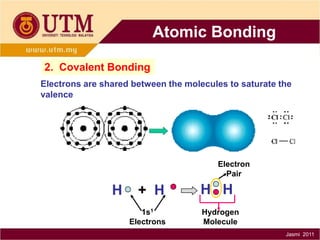
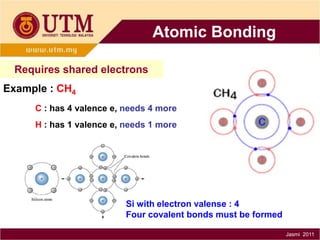
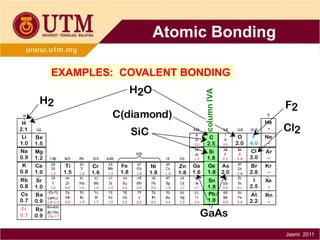
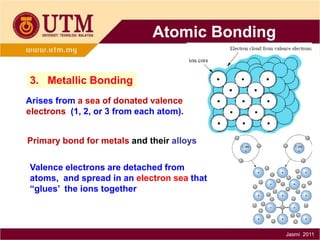
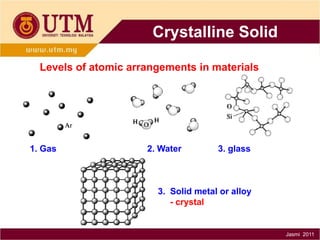
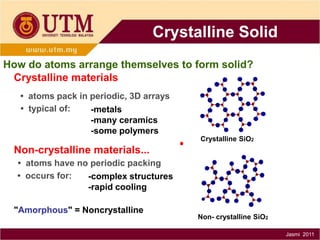
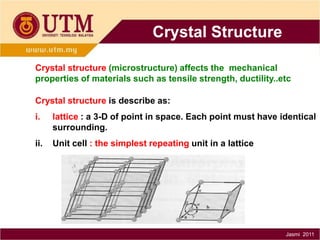
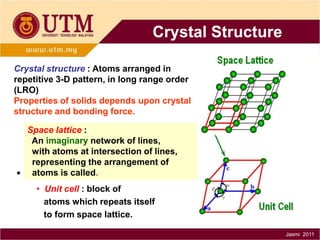
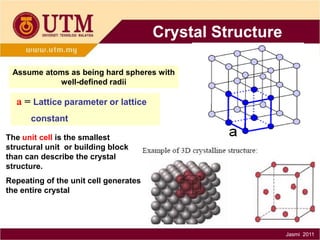
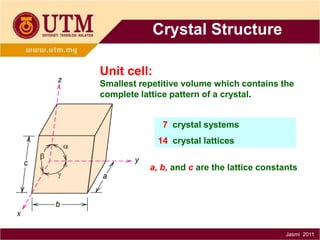
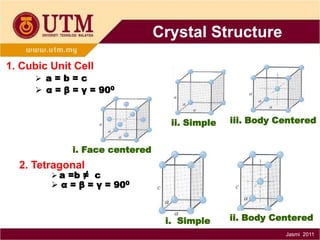
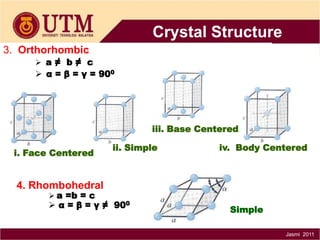
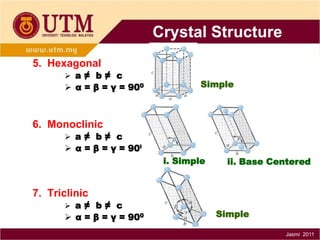
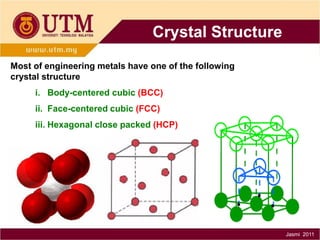
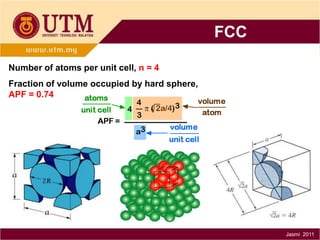
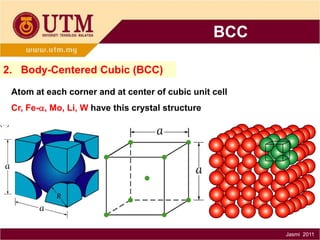
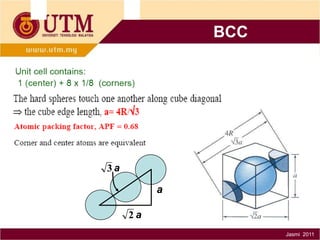
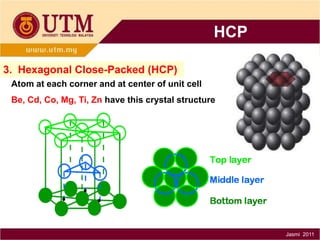
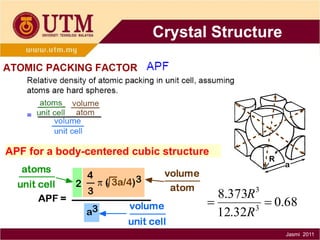
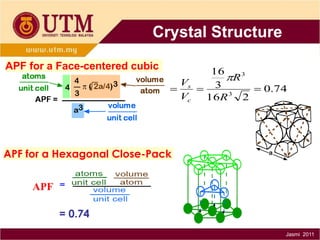
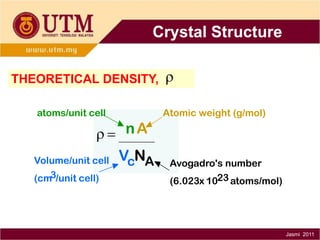
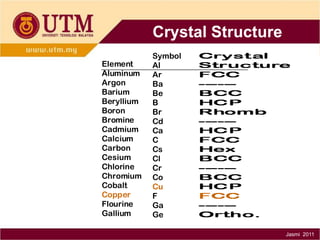
1. Materials science is the study of relationships between the structure and properties of materials. It relates how the atomic and molecular structure of a material influences its properties.
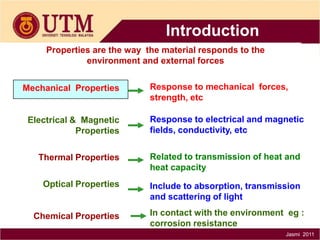
2. A material's properties determine how it responds to external forces and the environment. Key properties include mechanical, electrical, thermal, optical, and chemical properties. Mechanical properties describe response to forces like strength and toughness.
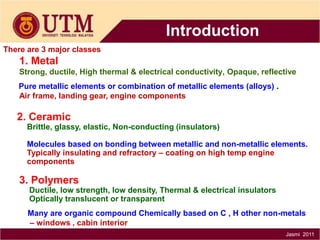
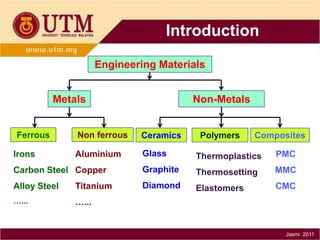
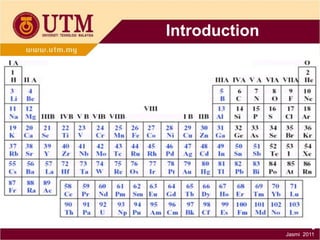
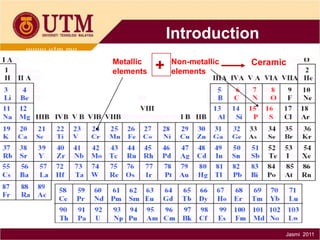
3. There are three main classes of materials: metals, ceramics, and polymers. Metals are strong, ductile, and conductive. Ceramics are brittle but heat resistant. Polymers are lightweight and insulating. Materials science helps understand materials and design new components.