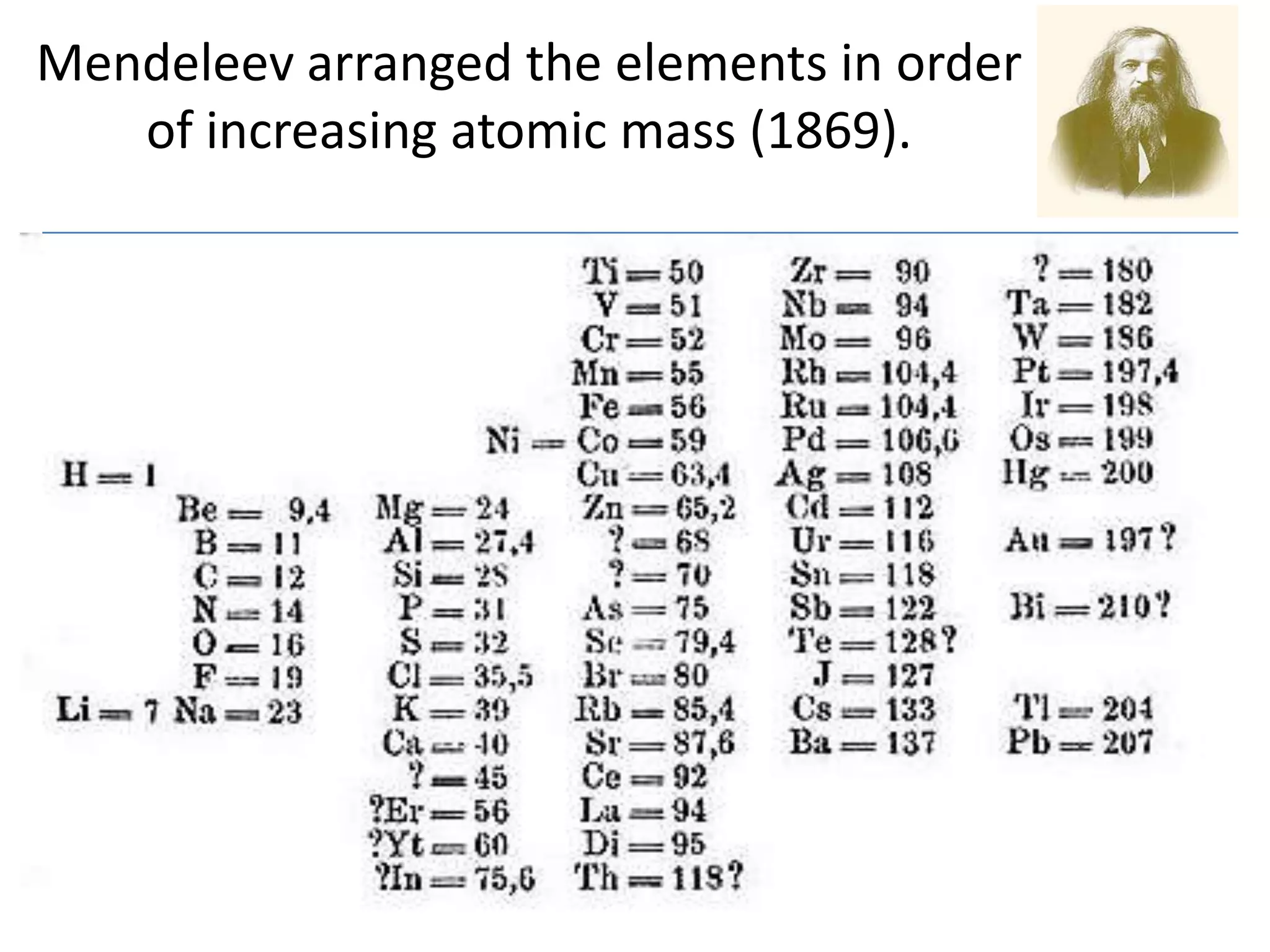
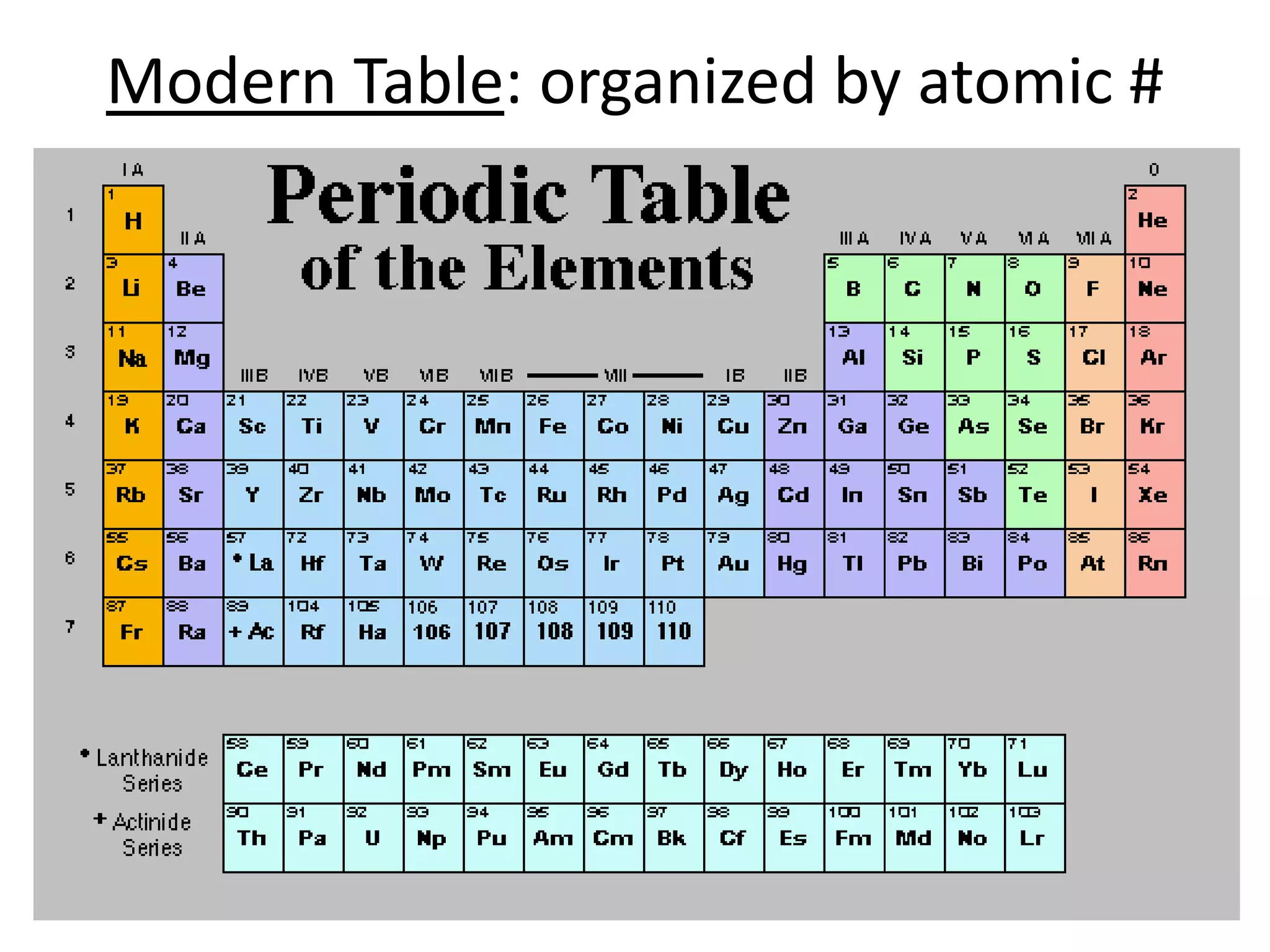
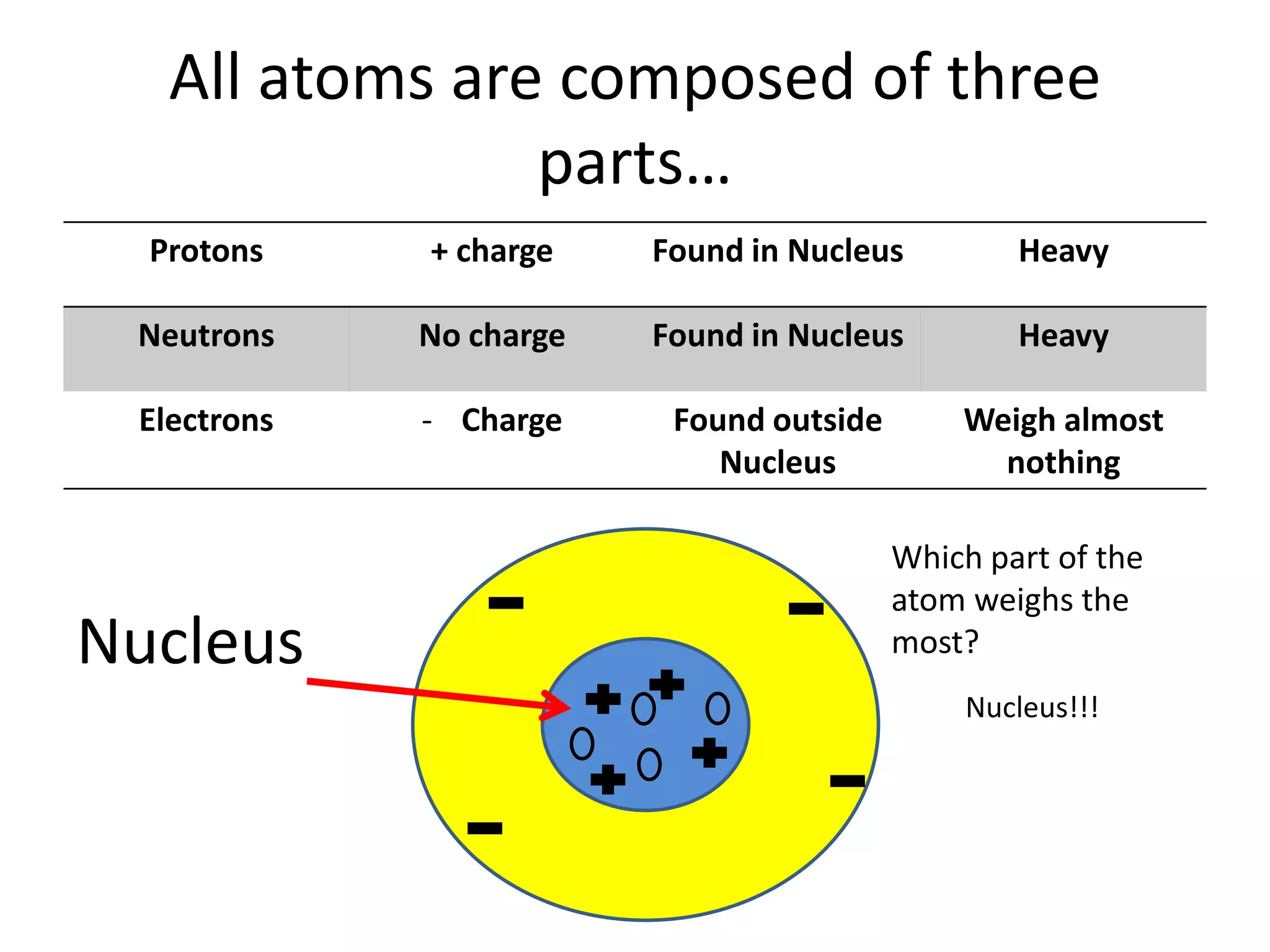
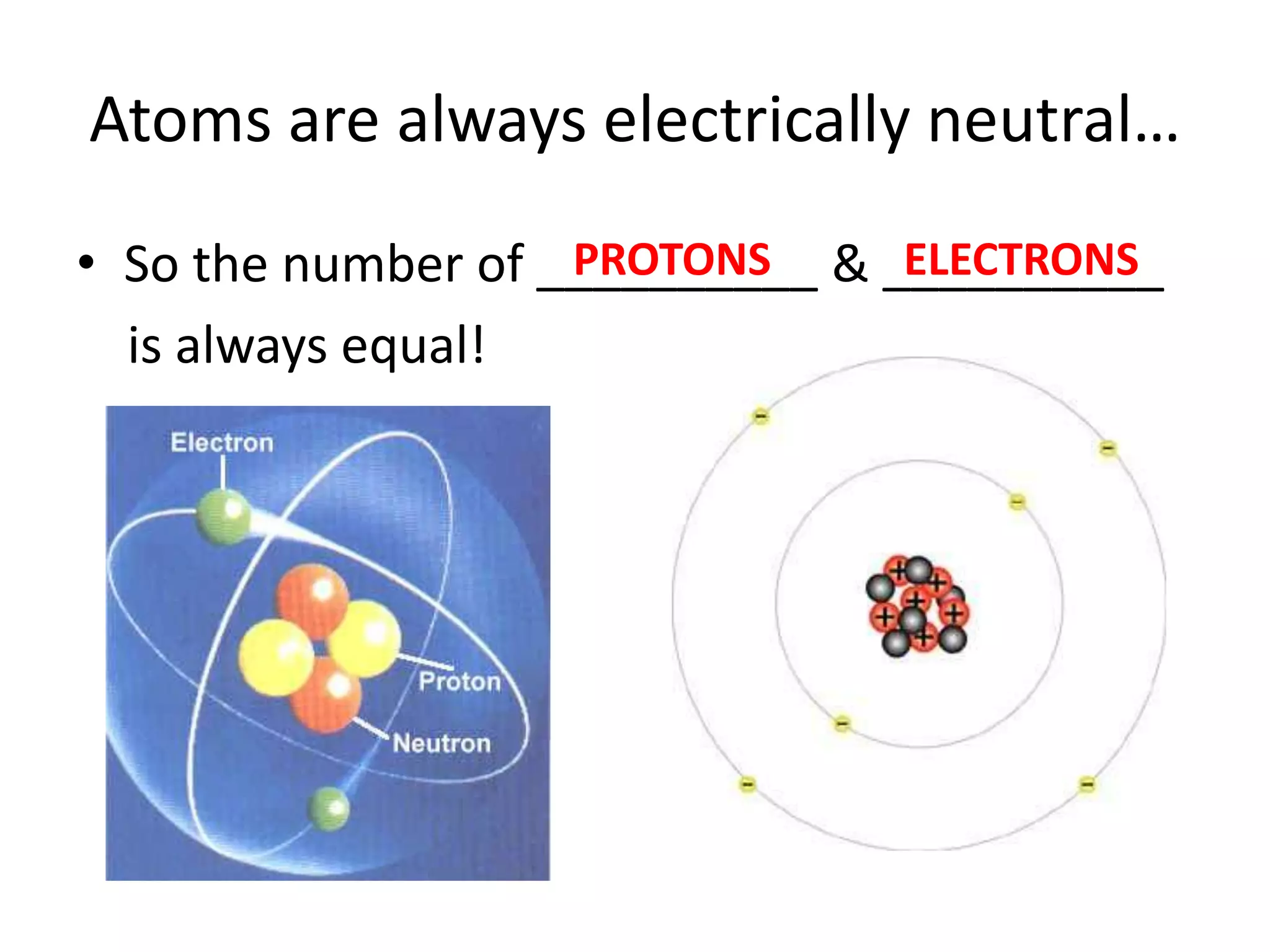
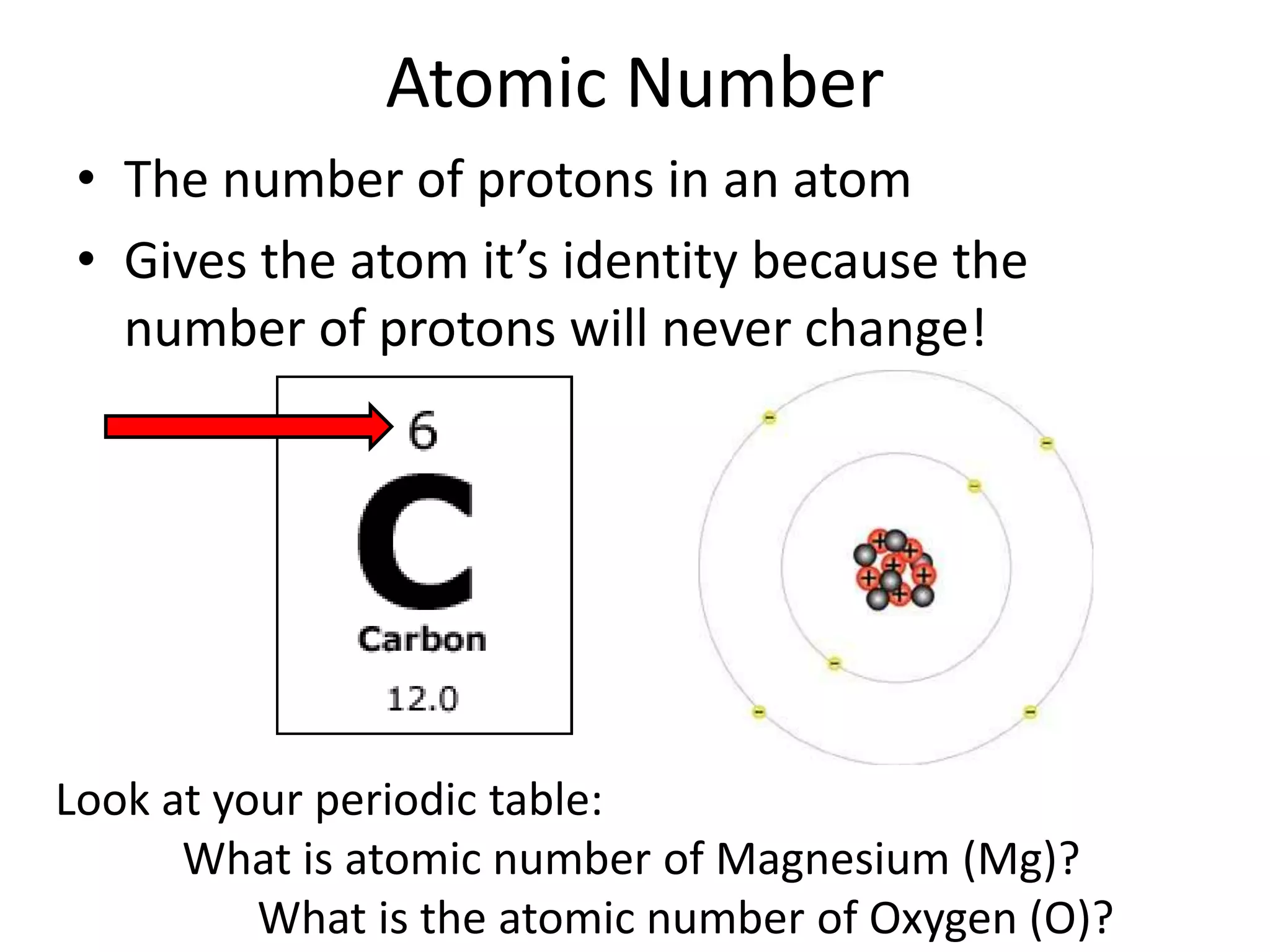
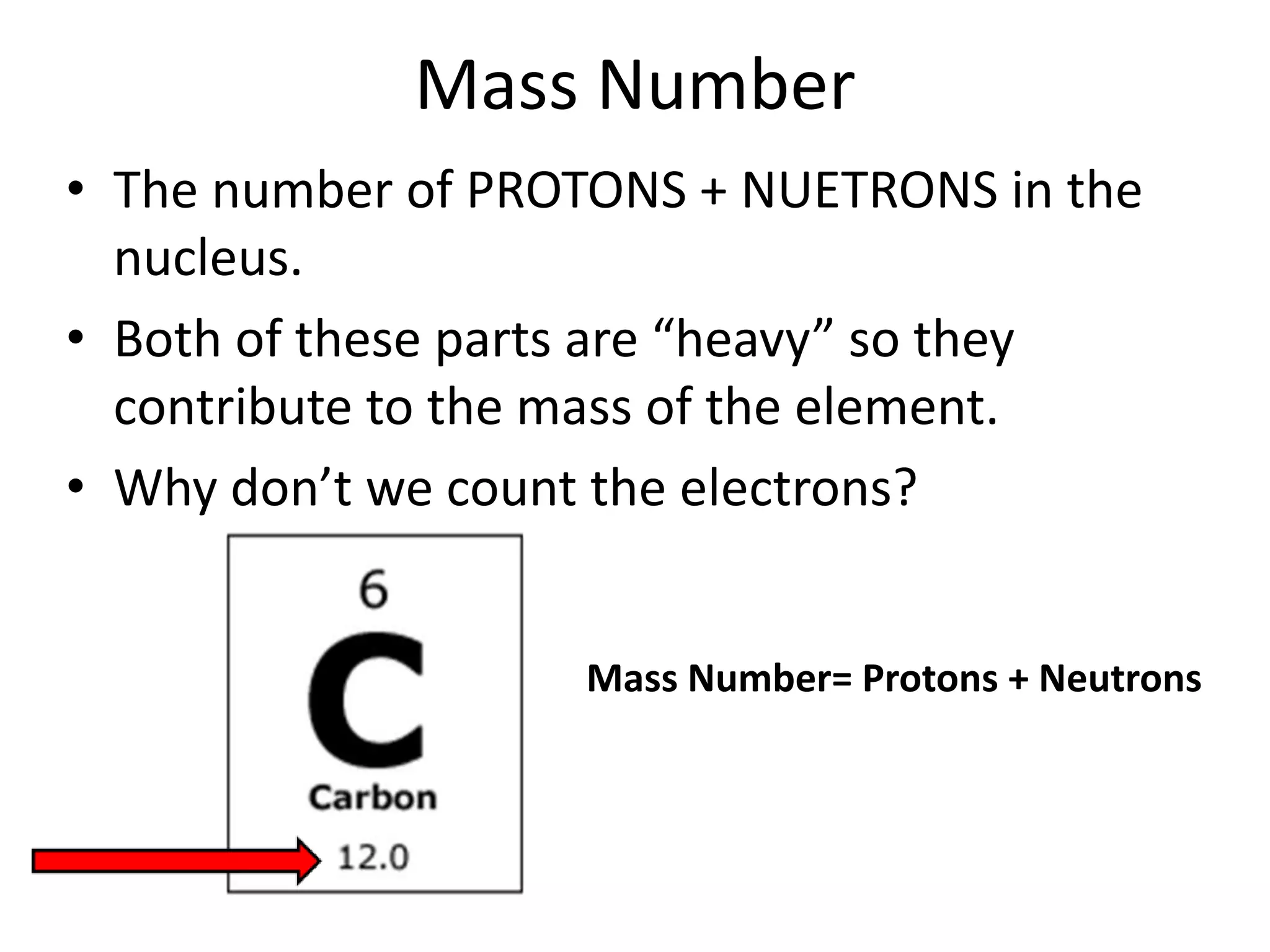
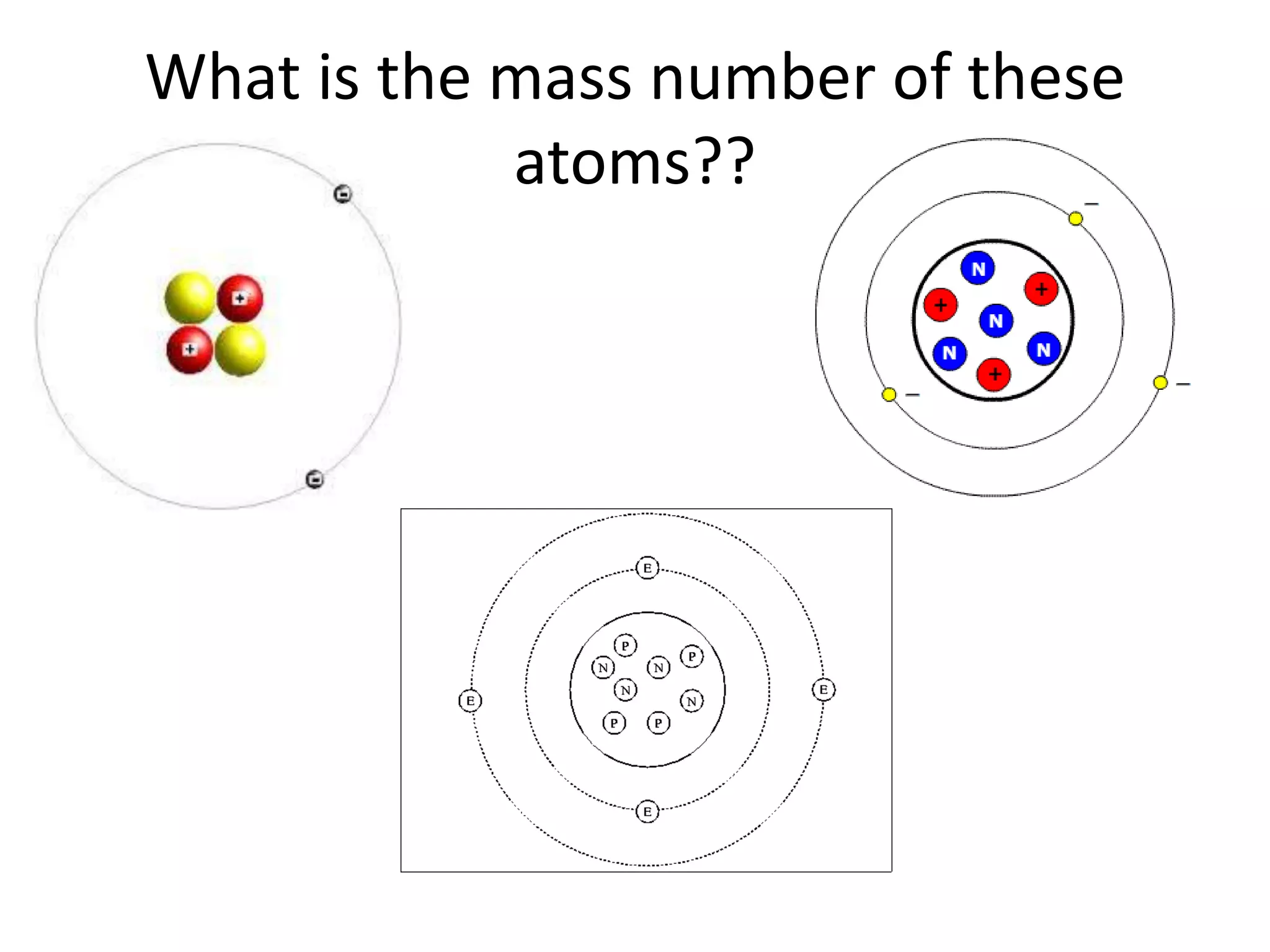
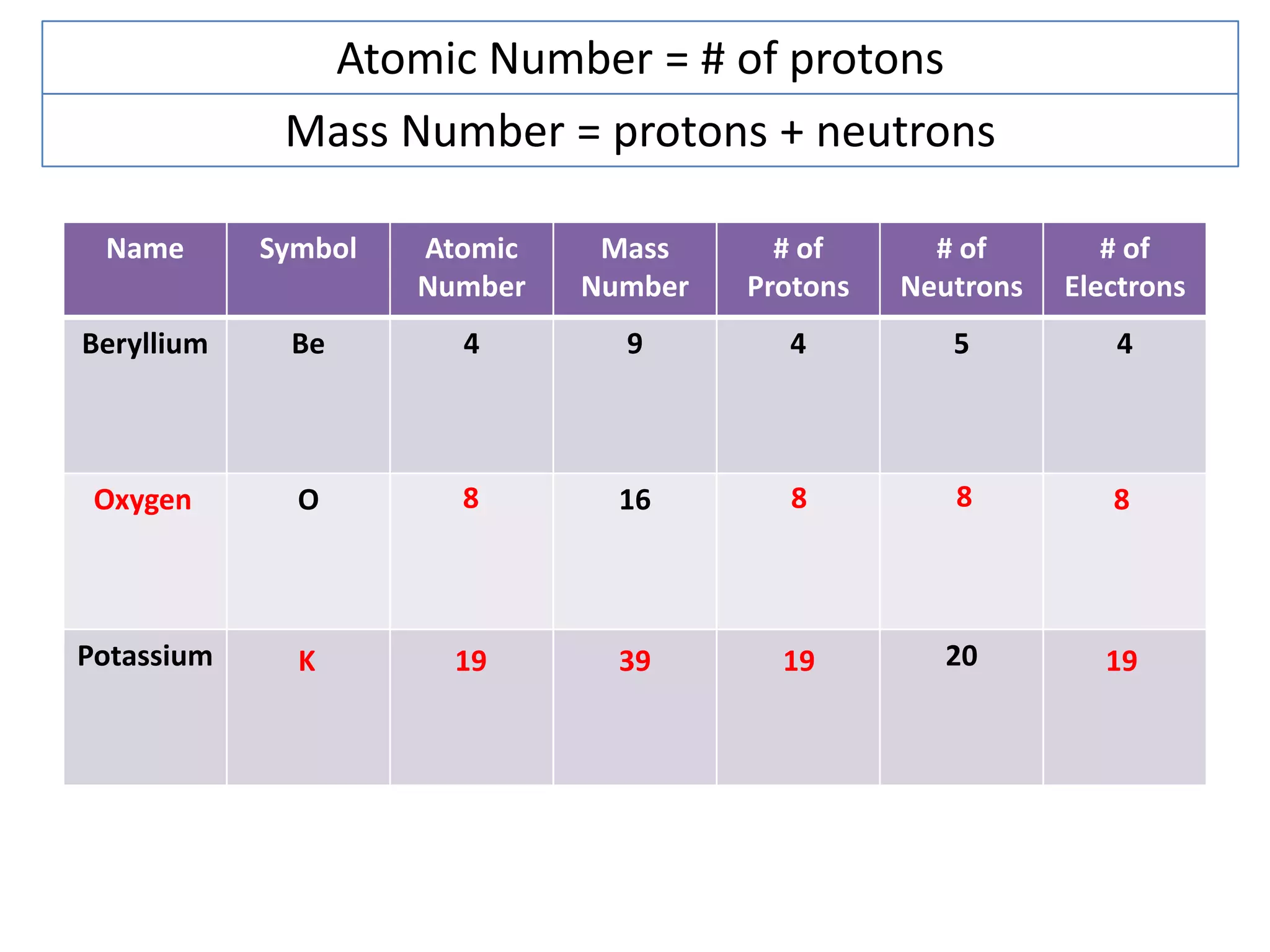
The document discusses atomic structure and the key parts of an atom: protons, neutrons, and electrons. It explains that protons are found in the nucleus and give an atom its atomic number. The number of protons never changes for a given element. It also defines atomic number as the number of protons and mass number as the total number of protons and neutrons in an atom's nucleus.