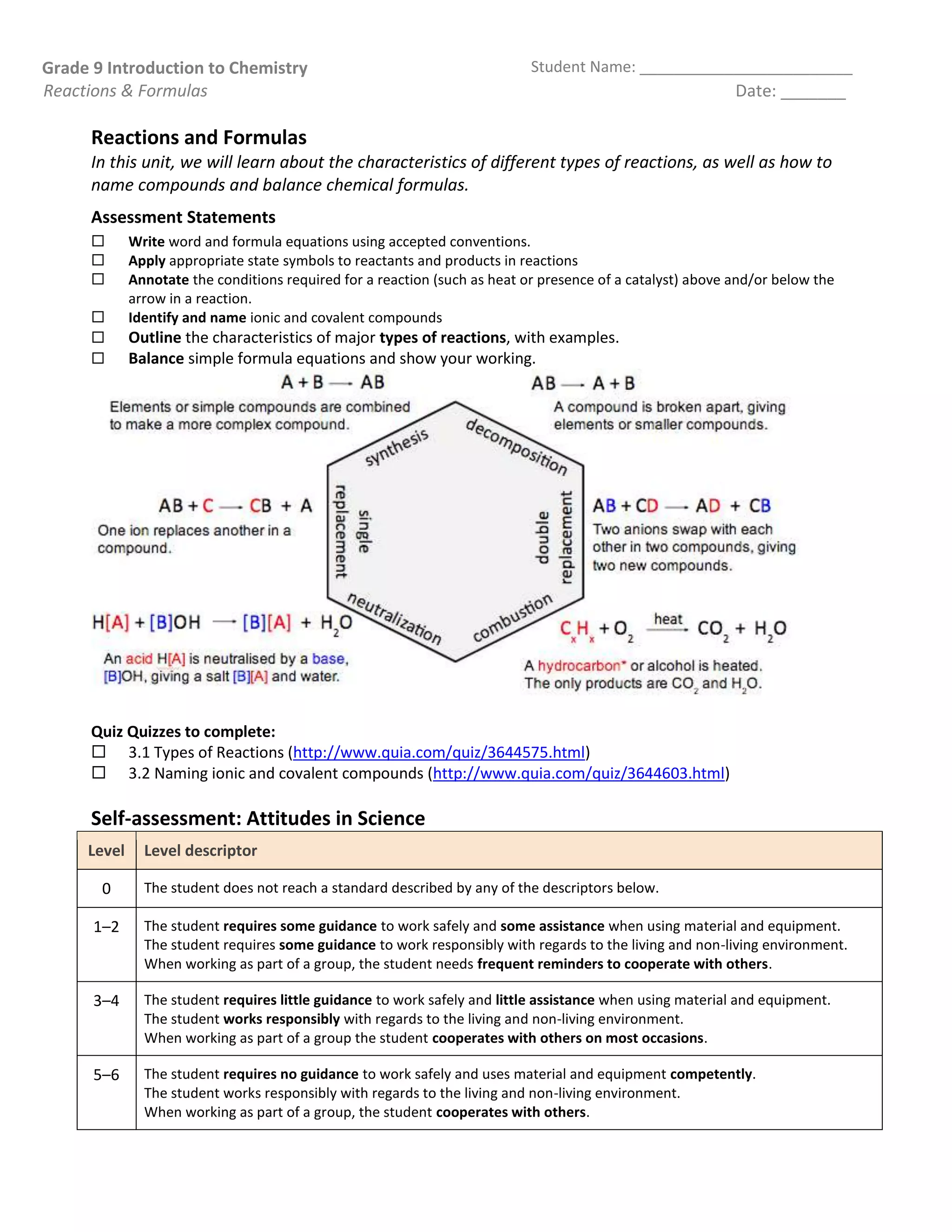
The document outlines key concepts in chemistry for grade 9, focusing on types of reactions, naming compounds, and balancing chemical equations. It covers synthesis, decomposition, combustion, single and double replacement, and neutralization reactions, along with safety precautions and methods for observing reactions. Additionally, it emphasizes the importance of the law of conservation of mass and provides self-assessment criteria for students.