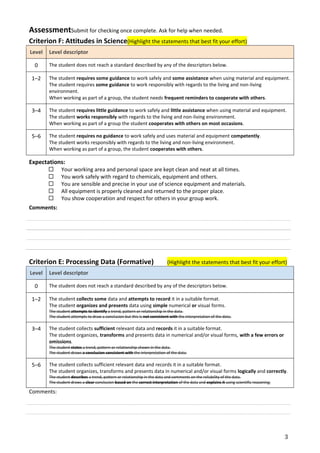
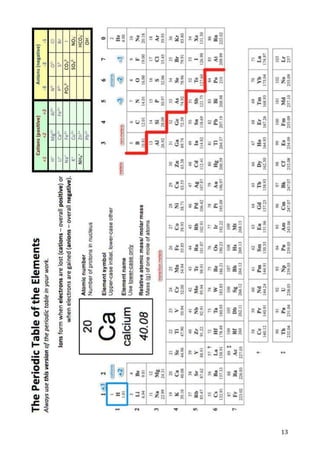
This document provides an introduction to working as a chemist. It outlines the scientific method and defines key terms like facts, laws, hypotheses, independent and dependent variables. It also lists the SI units used to measure common properties in chemistry like mass, length, temperature and concentration. Students are assessed on their understanding of concepts like accuracy, precision, significant figures and processing quantitative and qualitative data. Related concepts and terminology are defined to aid understanding.