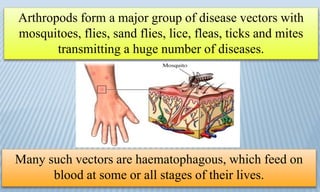
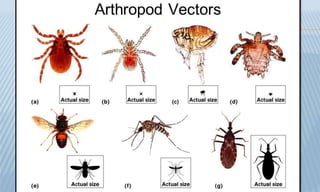
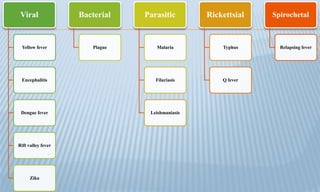
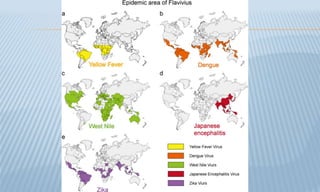
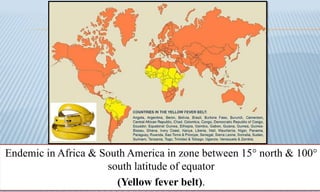
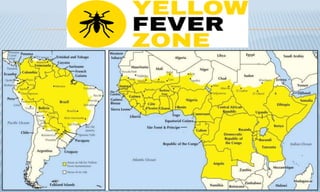
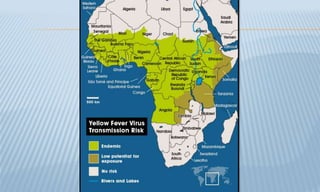
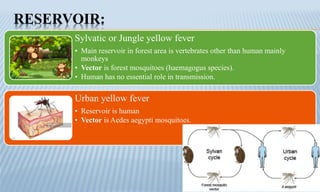
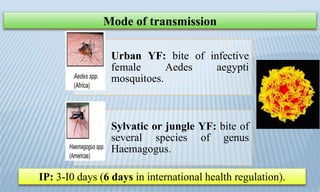
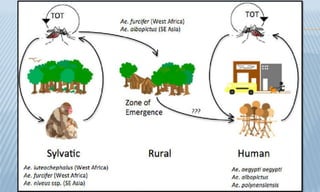
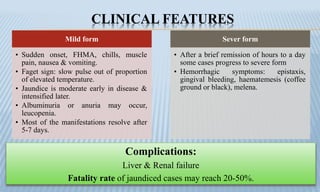
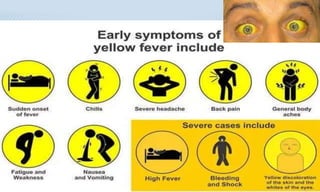
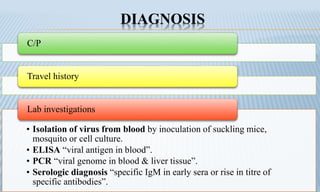
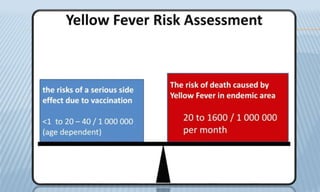
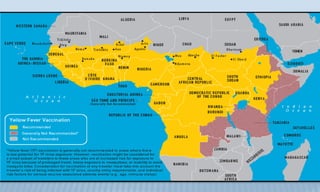
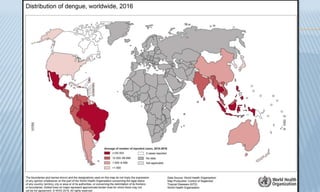
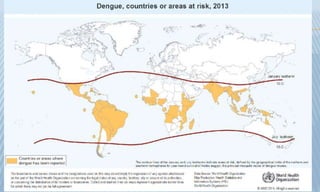
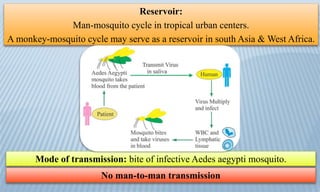
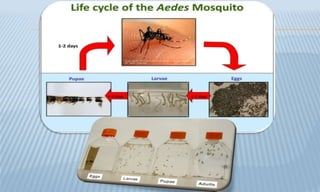
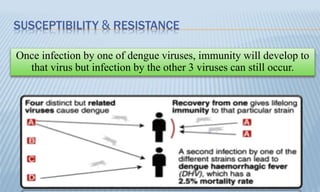
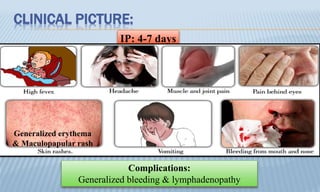
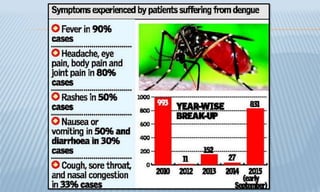
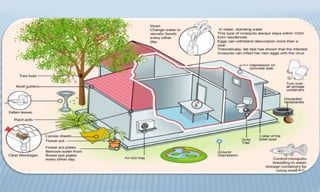
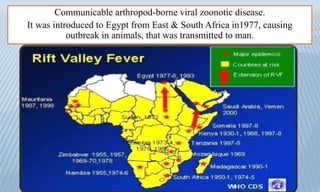
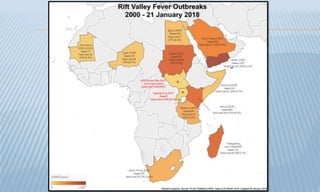
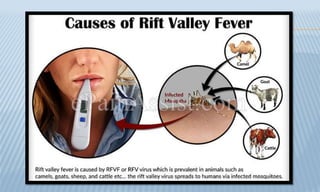
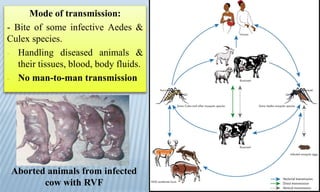
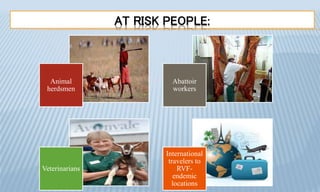
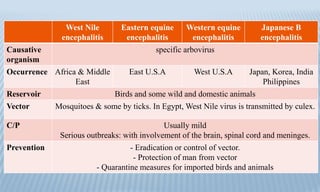
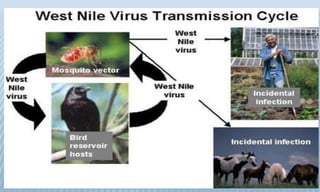
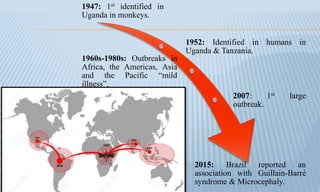
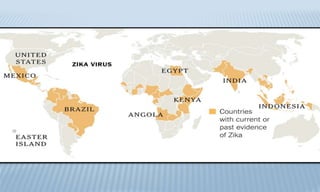
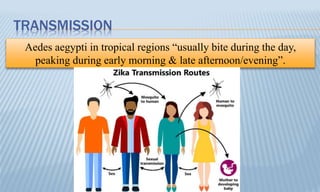
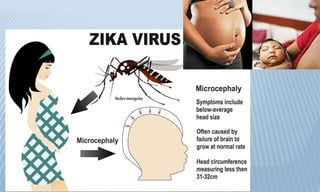
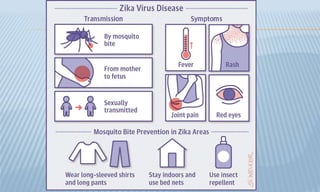
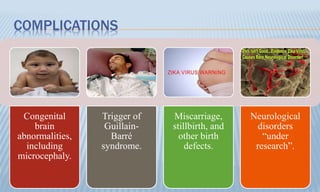
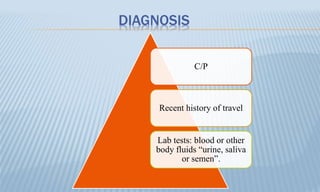
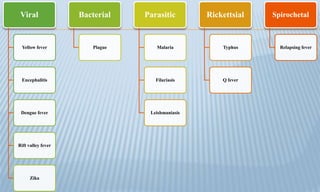
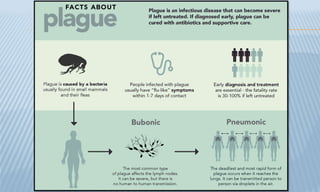
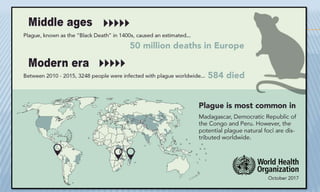
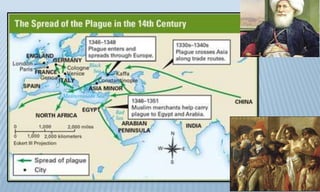
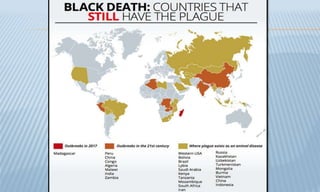
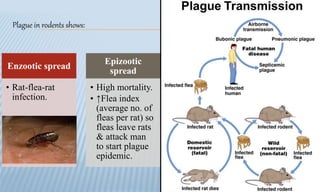
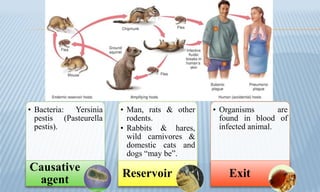
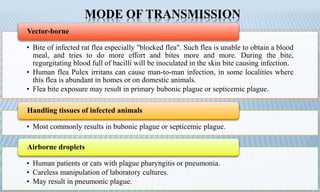
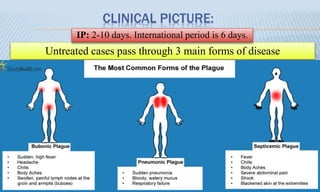
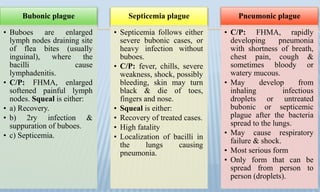
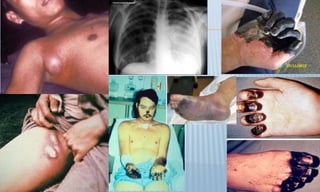
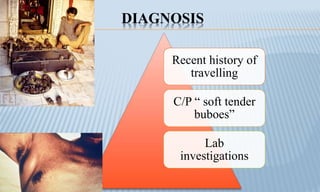
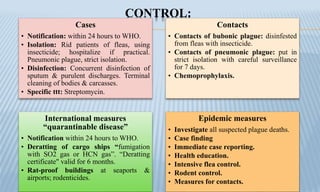
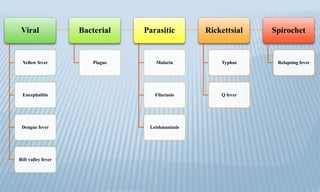
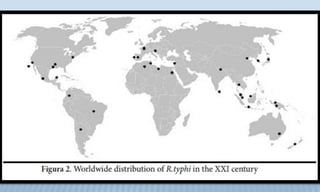
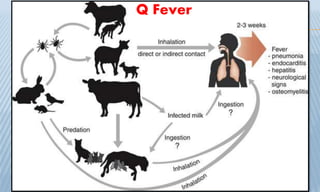
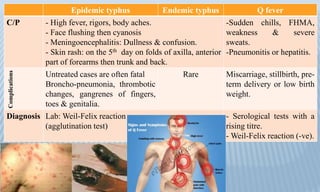
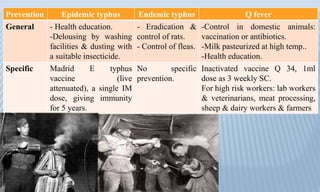
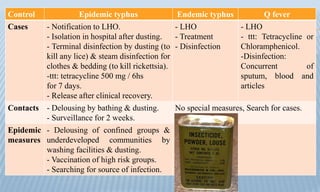
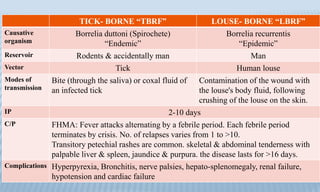
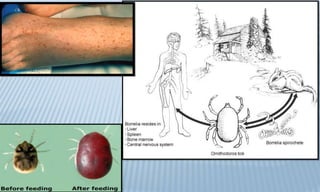
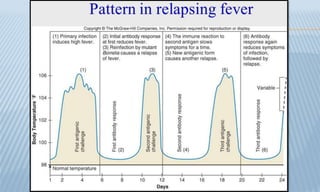
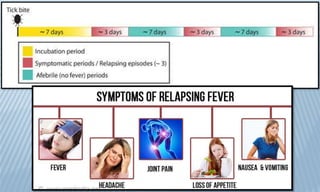
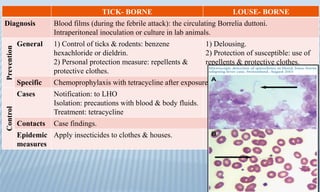
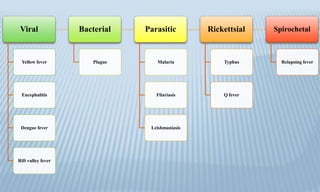
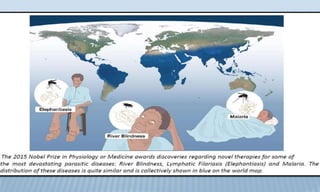
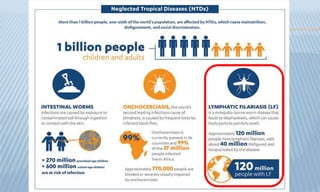
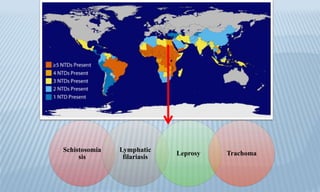
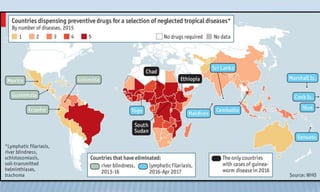
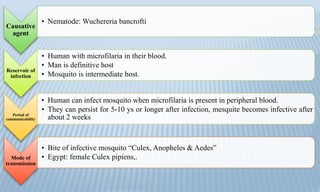
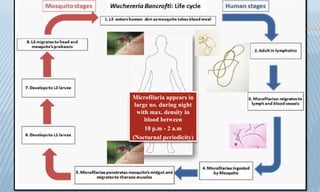
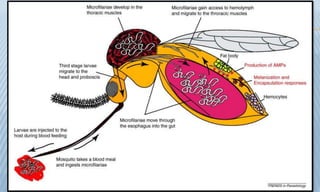
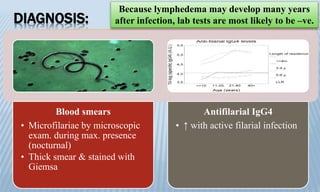
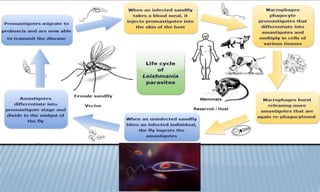
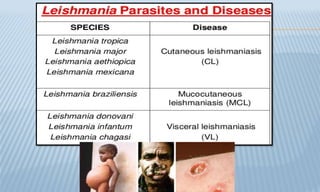
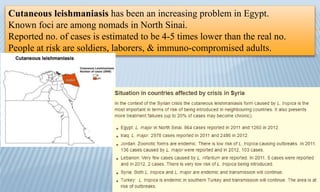
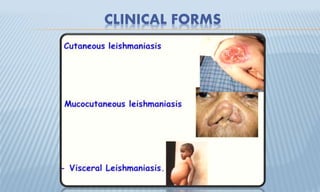
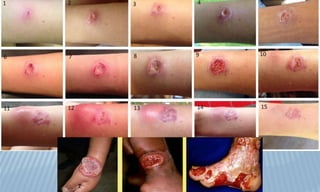
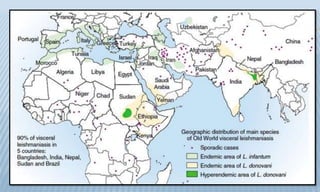
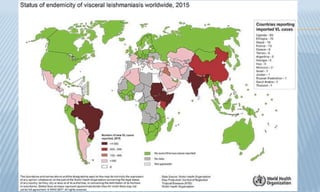
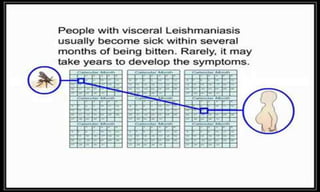
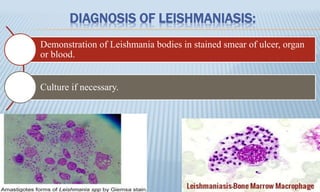
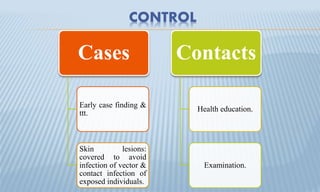
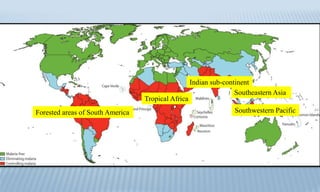
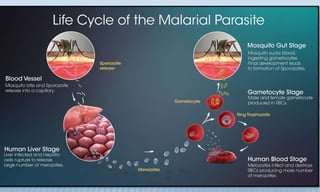
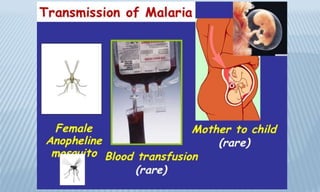
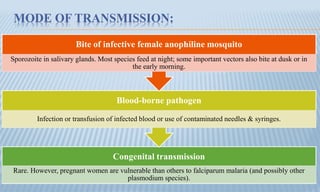
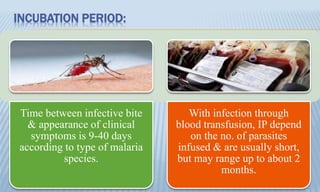
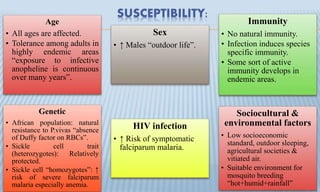
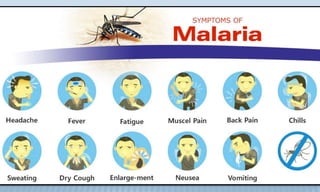
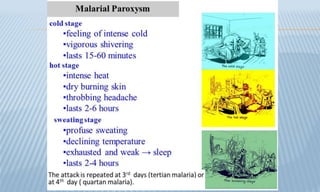
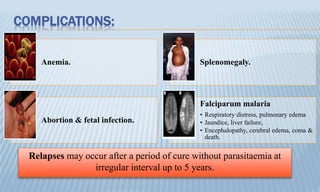
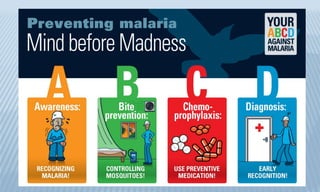
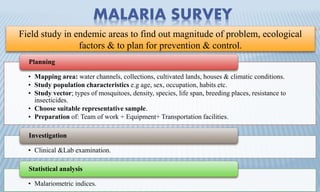
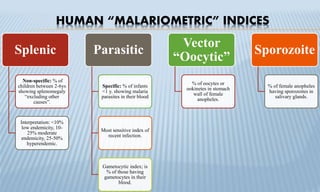
Arthropod-borne diseases are transmitted by vectors such as mosquitoes and ticks, causing illnesses like yellow fever, dengue, and rift valley fever. Each disease has specific transmission modes, clinical features, and prevention strategies, including vaccination and vector control. Methods for controlling these diseases involve public health measures, sanitation, and vaccination for at-risk populations.