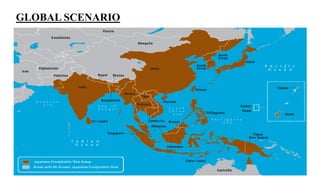
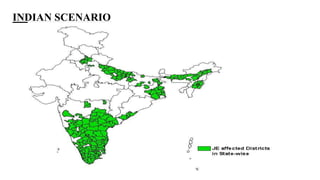
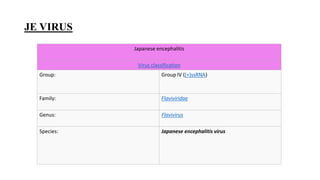
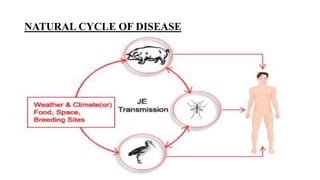
Japanese encephalitis is a mosquito-borne viral disease that is common in parts of Asia. It is transmitted to humans via bites from infected Culex mosquitoes. While most infections cause mild symptoms or no symptoms, approximately 1 in 250 infections result in encephalitis, which can be fatal in 30% of cases. Survivors often face permanent neurological impairments. Control efforts focus on vaccination programs and reducing mosquito populations in areas like rice paddies where they breed.