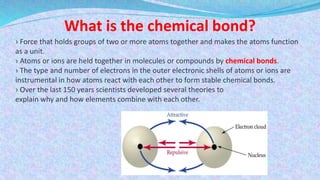
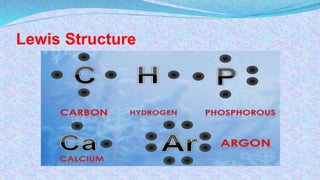
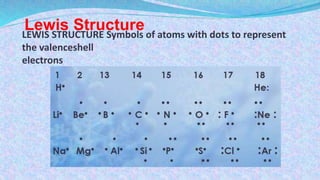
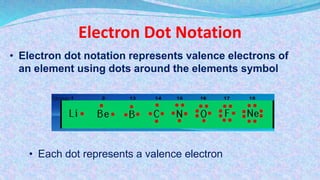
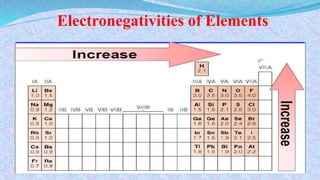
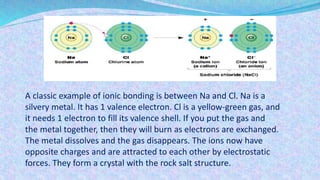
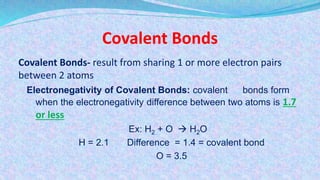
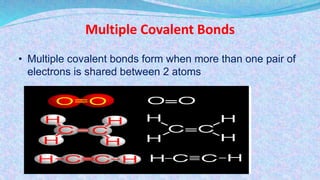
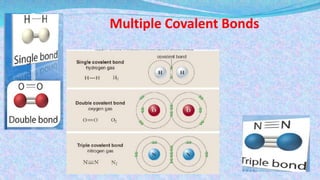
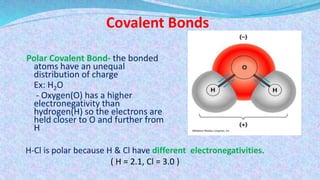
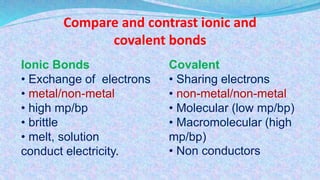
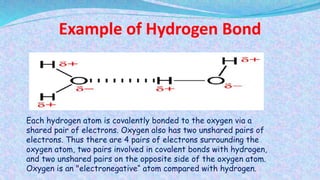
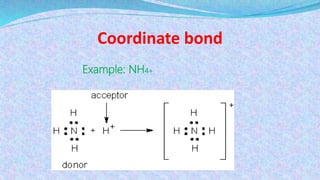
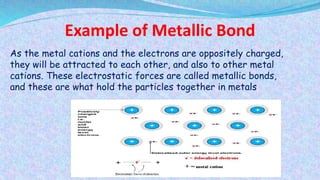
Chemical bonds are the forces that hold atoms together in molecules and compounds. There are several types of bonds including ionic bonds, covalent bonds, hydrogen bonds, metallic bonds, and coordinate bonds. Ionic bonds form between metals and nonmetals when electrons are transferred. Covalent bonds form when atoms share electrons. Hydrogen bonds occur between polar molecules containing hydrogen. Metallic bonds are electrostatic attractions between positively charged metal ions and delocalized electrons. Coordinate bonds form when both electrons in a bond come from the same atom. Each bond type influences the properties of the resulting compounds.