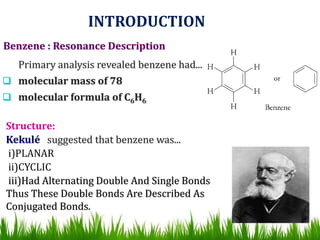
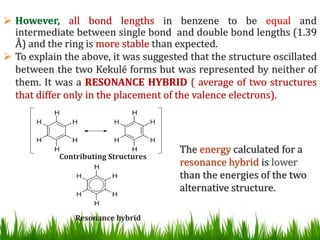
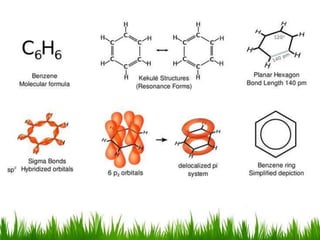
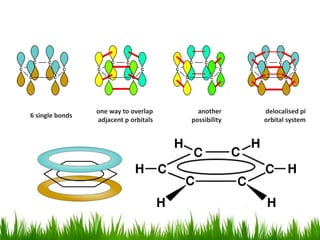
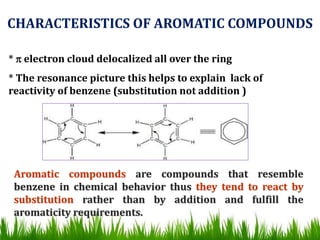
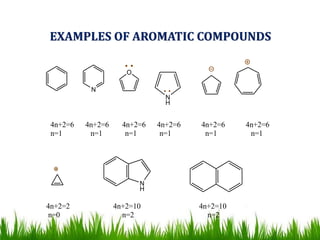
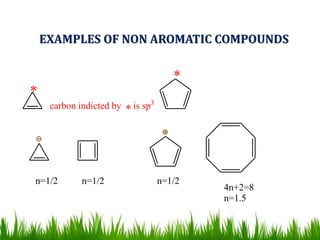
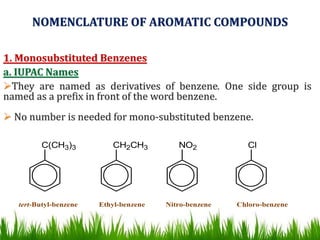
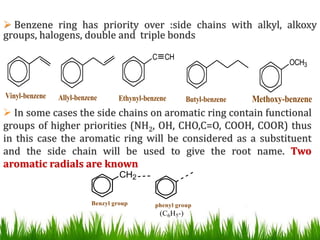
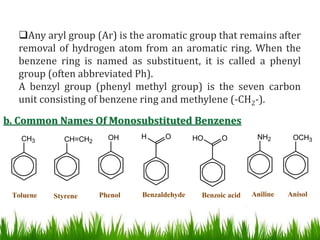
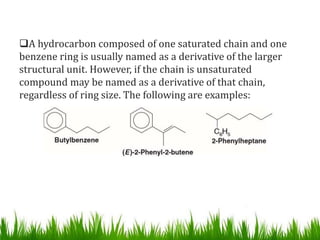
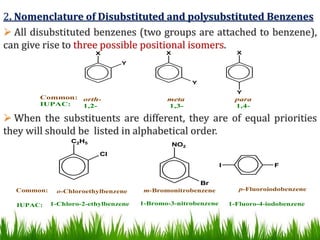
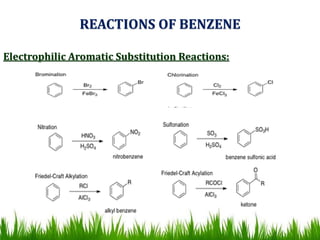
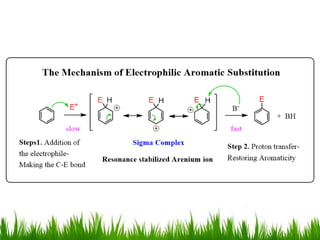
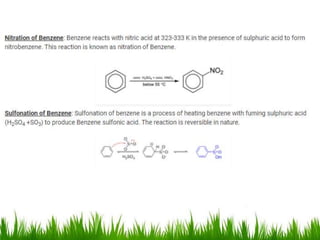
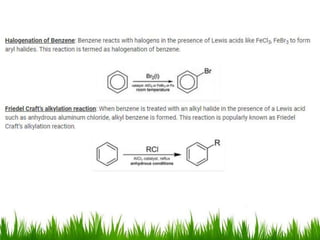
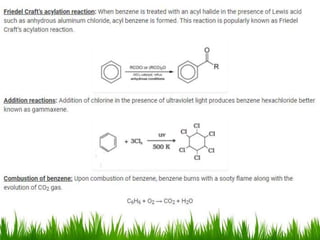
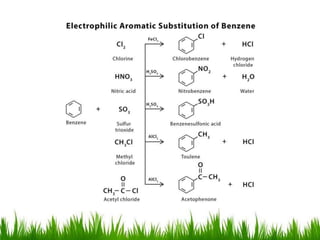
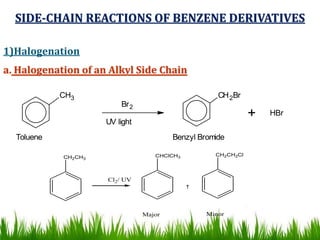
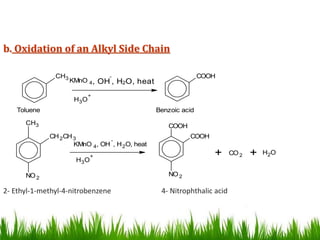
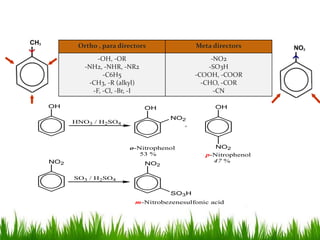
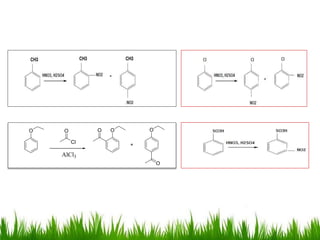
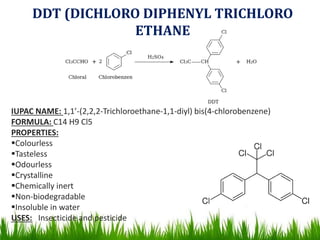
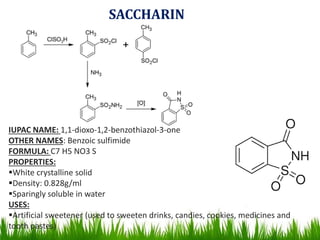
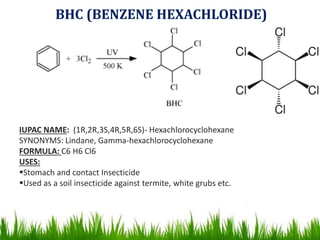
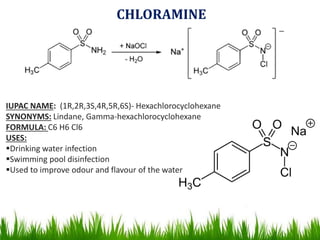
The document discusses the structure and properties of benzene. It explains Kekulé's suggestion that benzene has alternating double and single bonds in a planar cyclic structure. However, benzene's properties are better explained by the resonance hybrid model, where the pi electrons are delocalized around the ring. Aromatic compounds have delocalized pi electrons in a cyclic planar structure according to Hückel's rule of 4n+2 pi electrons. Examples of aromatic and non-aromatic compounds are given. The document also discusses the nomenclature, reactions, and properties of aromatic compounds including electrophilic aromatic substitution.