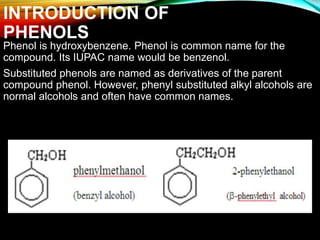
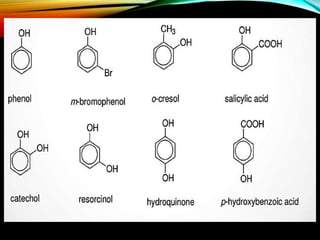
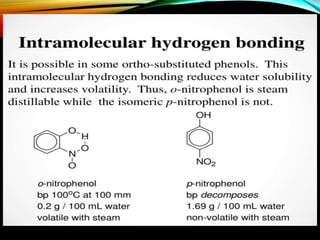
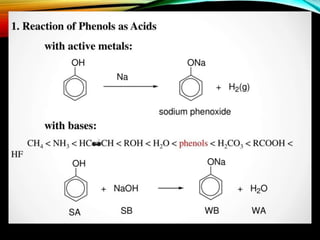
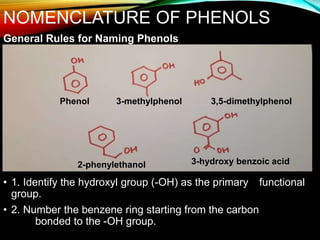
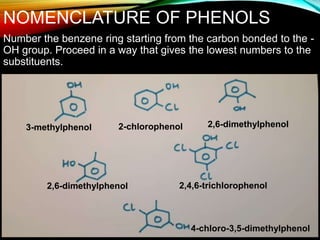
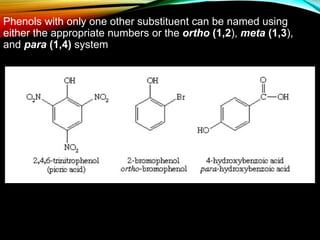
Phenols are organic compounds featuring a hydroxyl group bonded to an aromatic hydrocarbon, and they are used in industries ranging from pharmaceuticals to cosmetics. Discovered in 1834, phenol has various sources, including natural occurrences in plants and synthetic production from coal tar, and it has distinct physical properties such as high boiling and melting points due to strong hydrogen bonding. However, phenols are toxic and can affect health and the environment, necessitating safety measures during use and disposal.