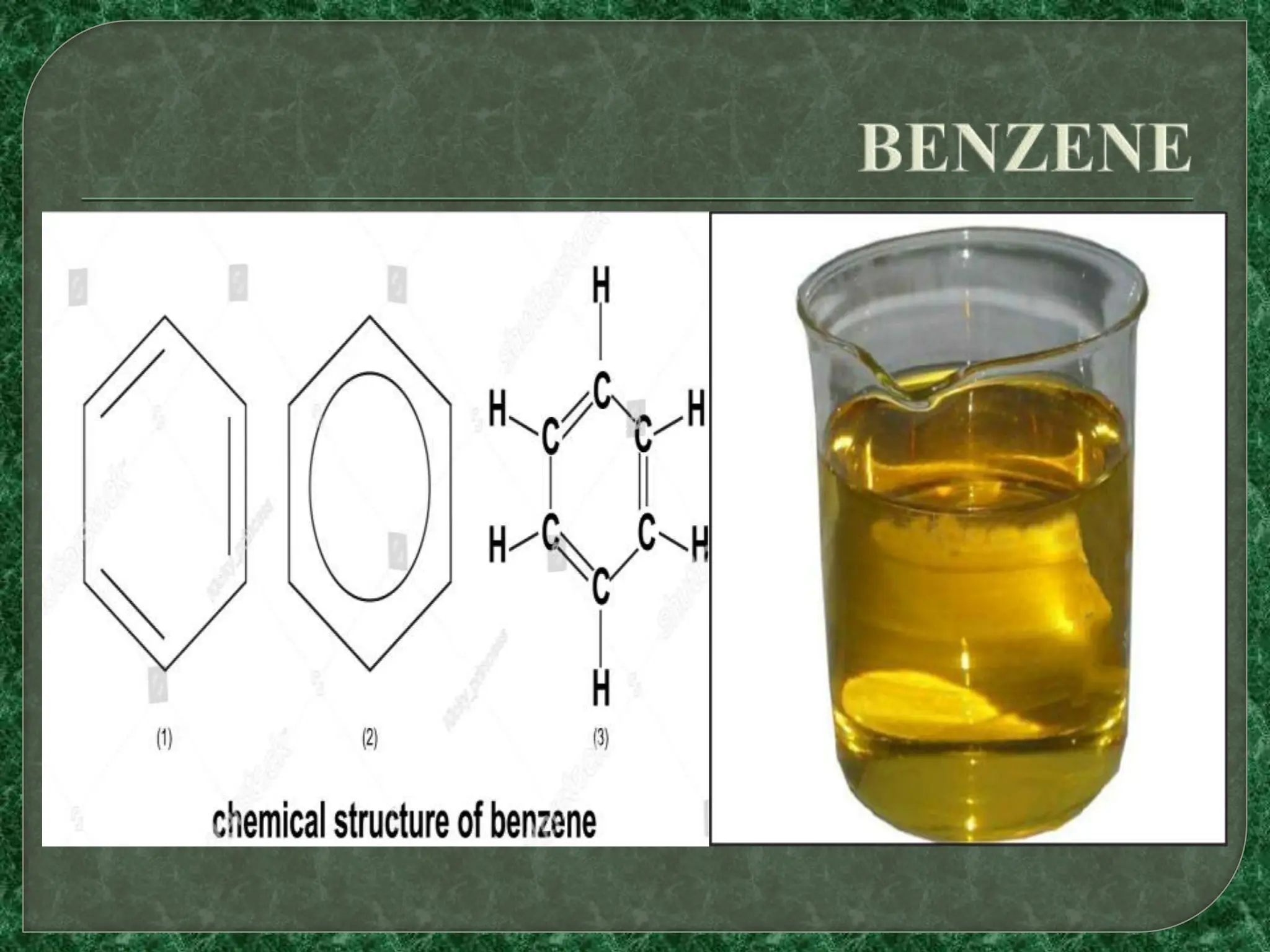
Benzene is the simplest aromatic hydrocarbon, with a six carbon ring structure and alternating double bonds. It is naturally produced from volcanoes and forest fires but is also a major industrial chemical made from coal and oil. Benzene is highly toxic and carcinogenic. It has many industrial uses such as in the production of pesticides, resins, detergents, synthetic fibers, plastics, and drugs. Domestically, benzene is used in glues, cleaners, and tobacco smoke, among other applications.





















![THE CRITERIA FOR AROMATICITY—
Four structural criteria must be satisfied for a compound to be aromatic.
Hückel’s Rule:
[1] A molecule must be cyclic. To be aromatic, each p orbital must overlap with
p orbitals on adjacent atoms.
[2] A molecule must be planar. All adjacent p orbitals must be aligned so that
the electron density can be delocalized.
[3] Each atom in an aromatic ring has a p orbital. These p orbitals must be
parallel so that a continuous overlap is possible around the ring.
[4] A molecule must satisfy Hückel’s rule, and contain a particular number of
electrons.
Benzene is aromatic and especially stable because it contains 6 electrons.](https://image.slidesharecdn.com/benzeneanditsderivatives-231006170836-fefd149a/75/BENZENE-AND-ITS-DERIVATIVES-pptx-23-2048.jpg)







