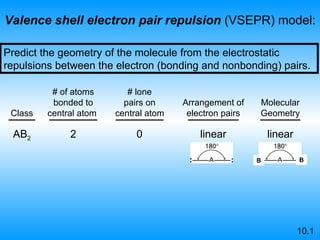
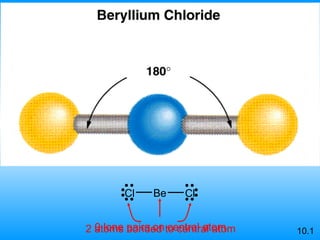
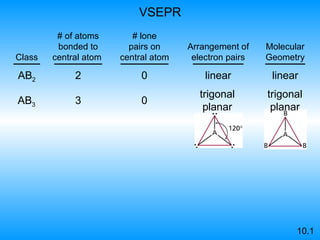
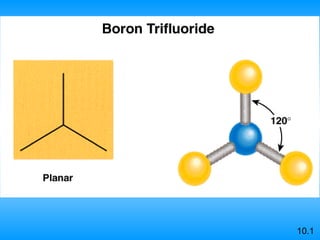
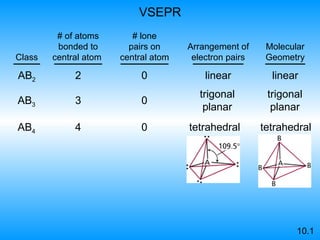
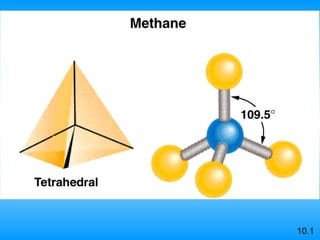
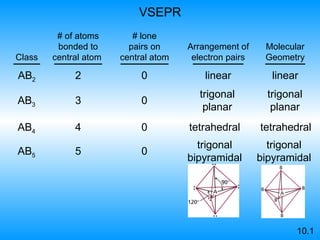
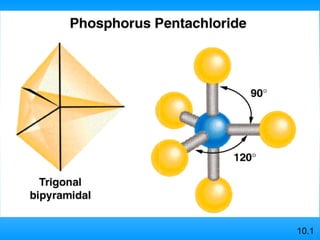
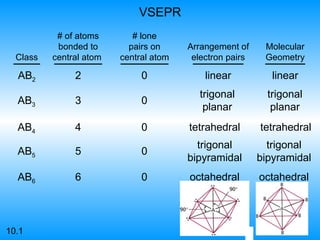
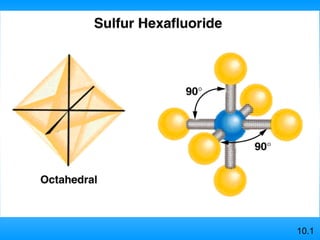
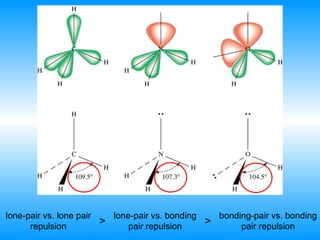
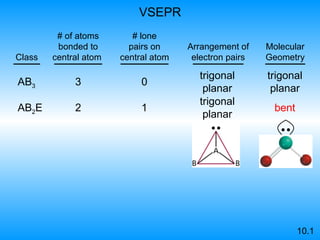
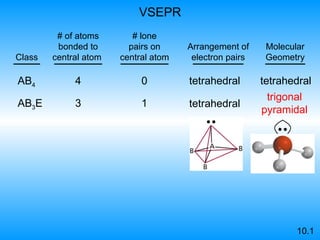
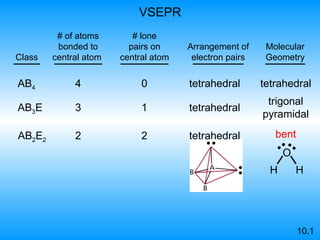
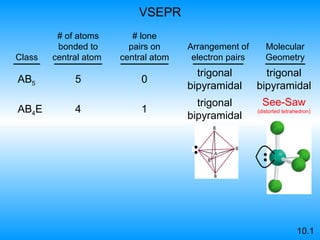
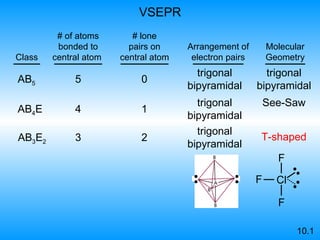
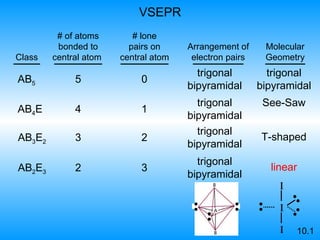
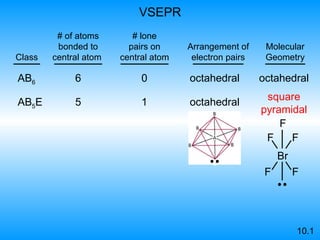
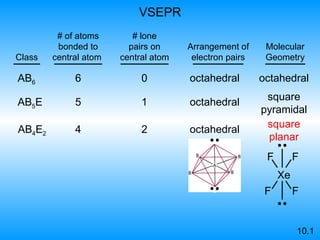
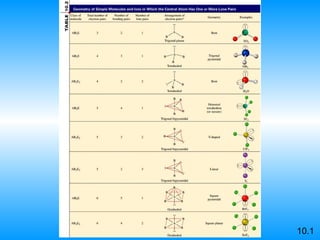
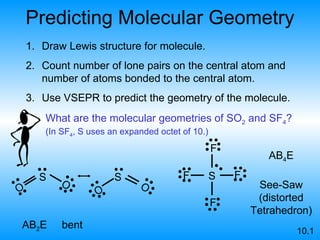
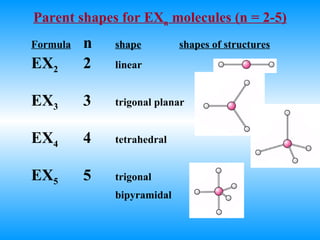
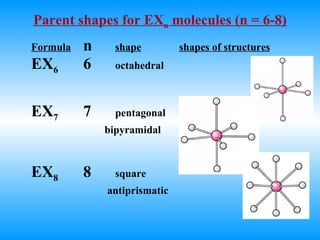
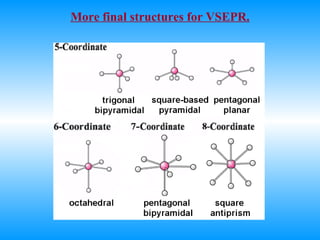
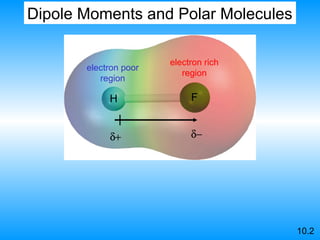
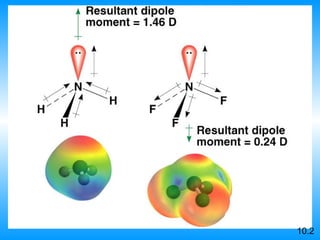
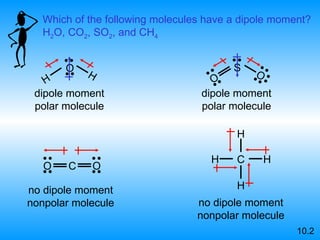
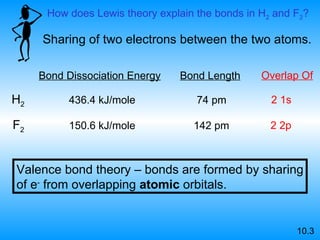
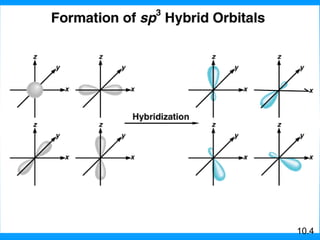
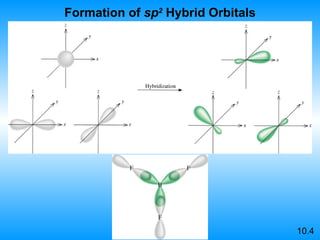
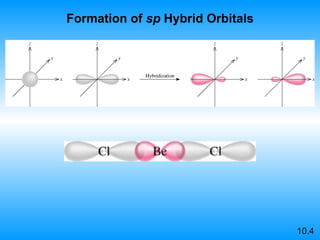
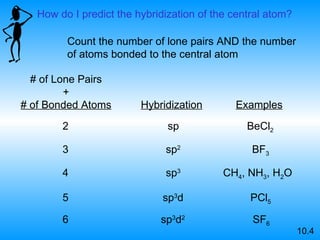
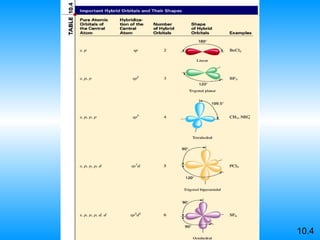
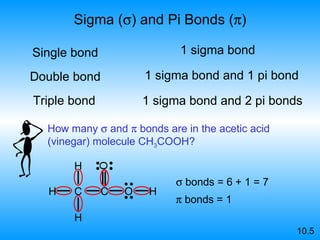
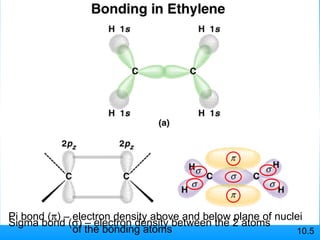
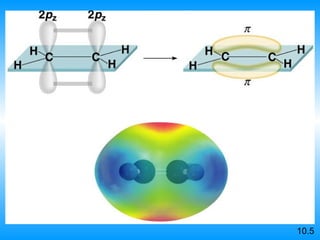
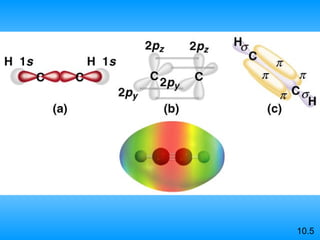
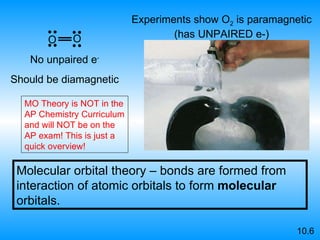
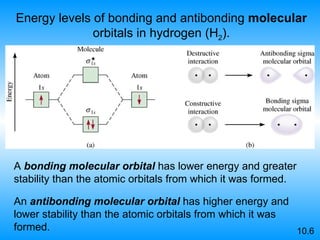
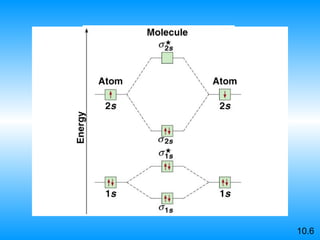
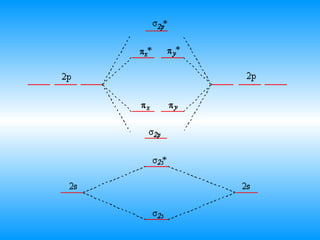
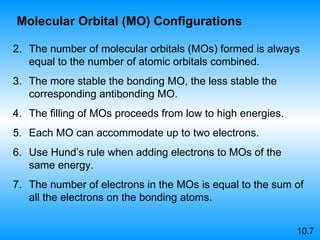
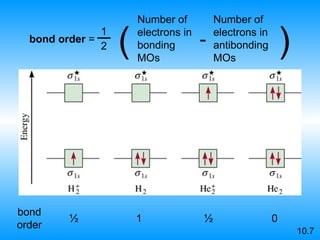
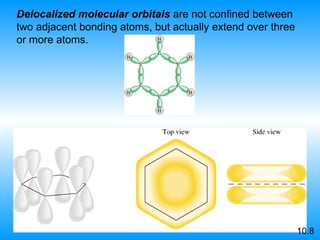
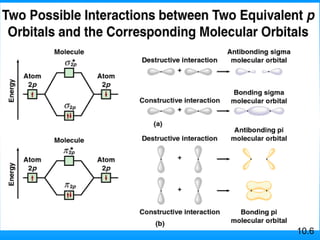
The document discusses molecular geometry and hybridization of atomic orbitals. It explains that the valence shell electron pair repulsion (VSEPR) model is used to predict molecular geometry based on electron pair repulsions. Molecular geometry is determined by the number of atoms bonded to the central atom and any lone pairs. Hybridization, such as sp, sp2, and sp3, is used to explain bonding based on the number of electron pairs around the central atom. Sigma and pi bonds are discussed, along with how hybridization relates to molecular geometry.