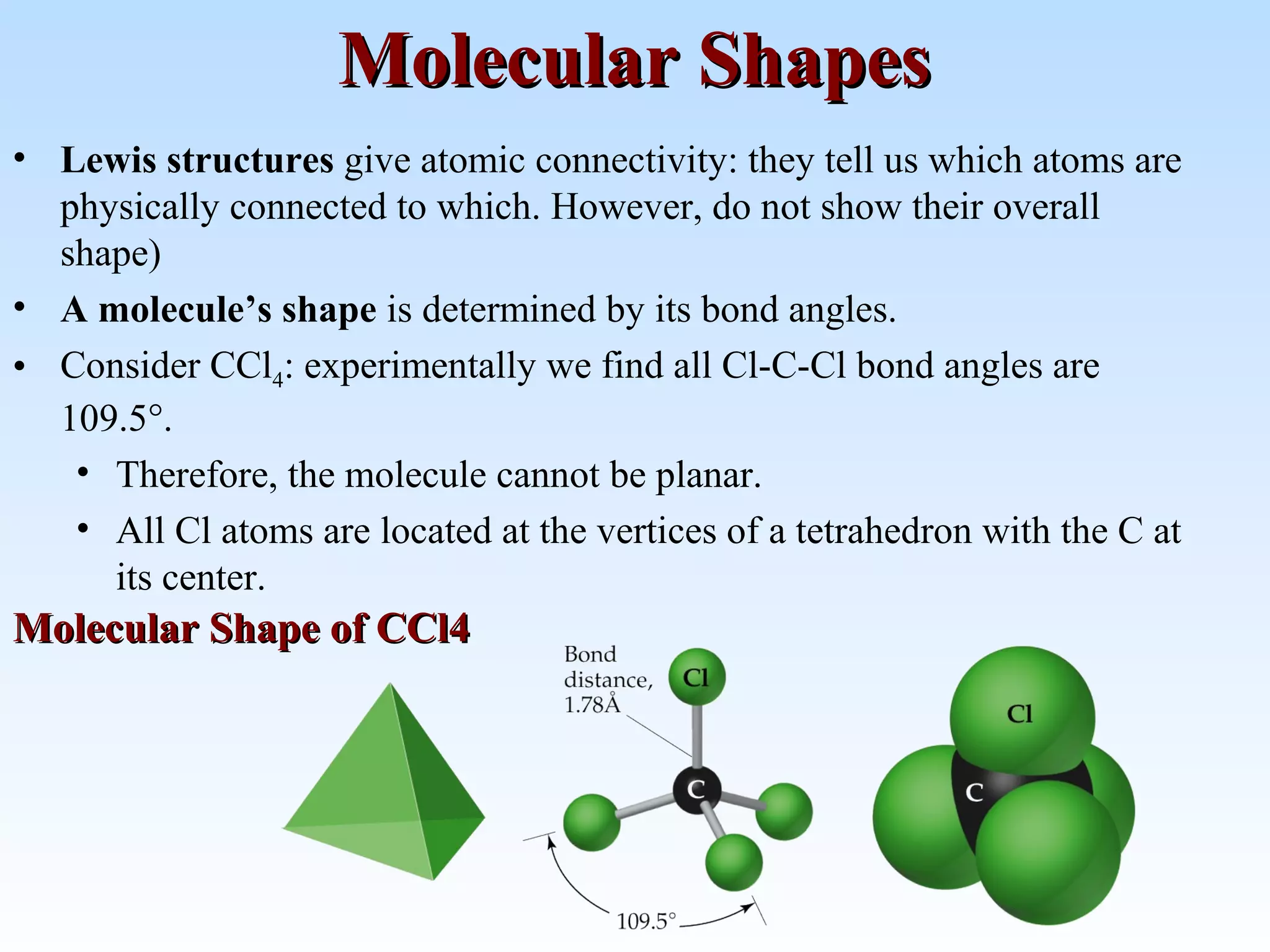
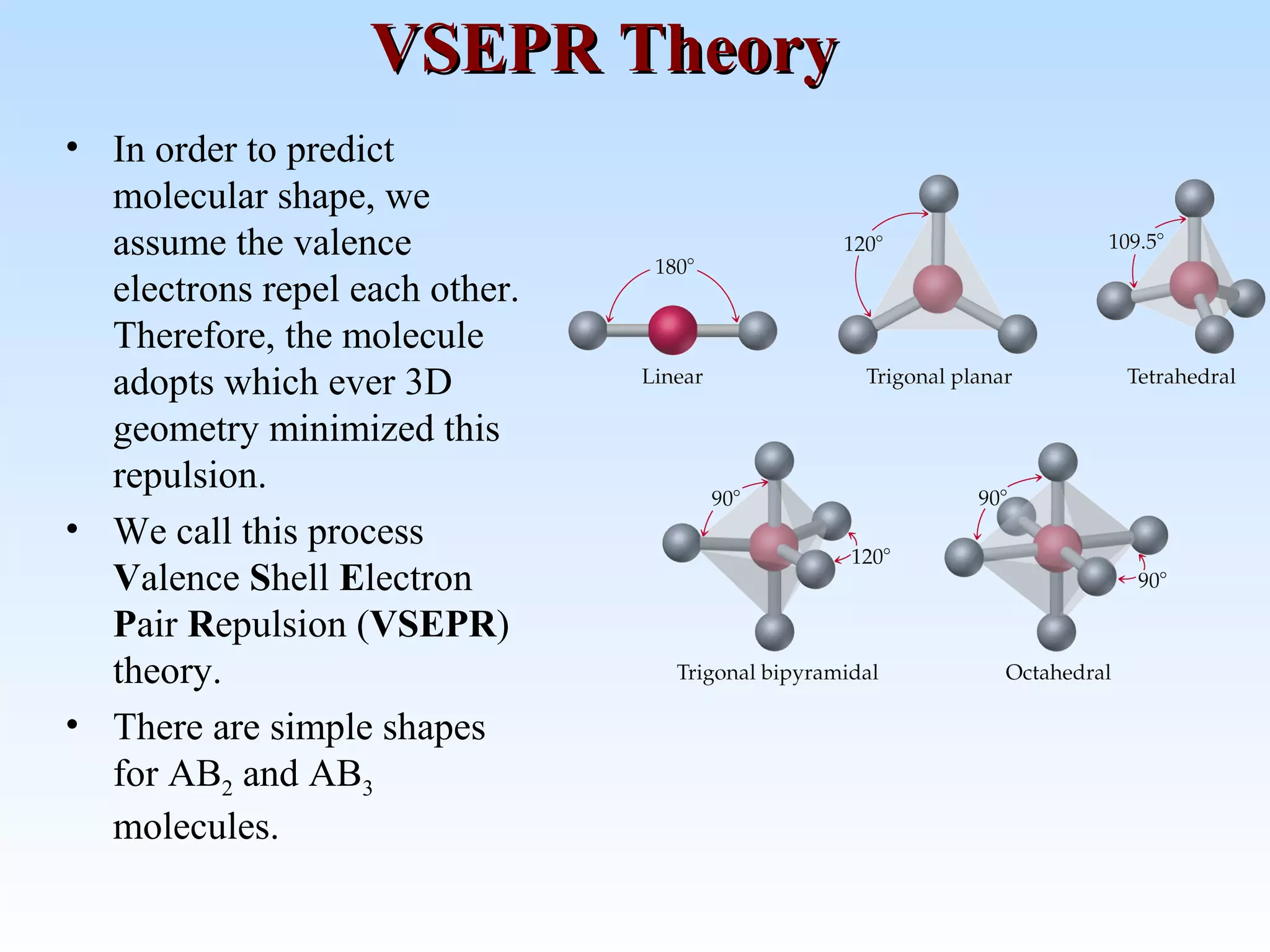
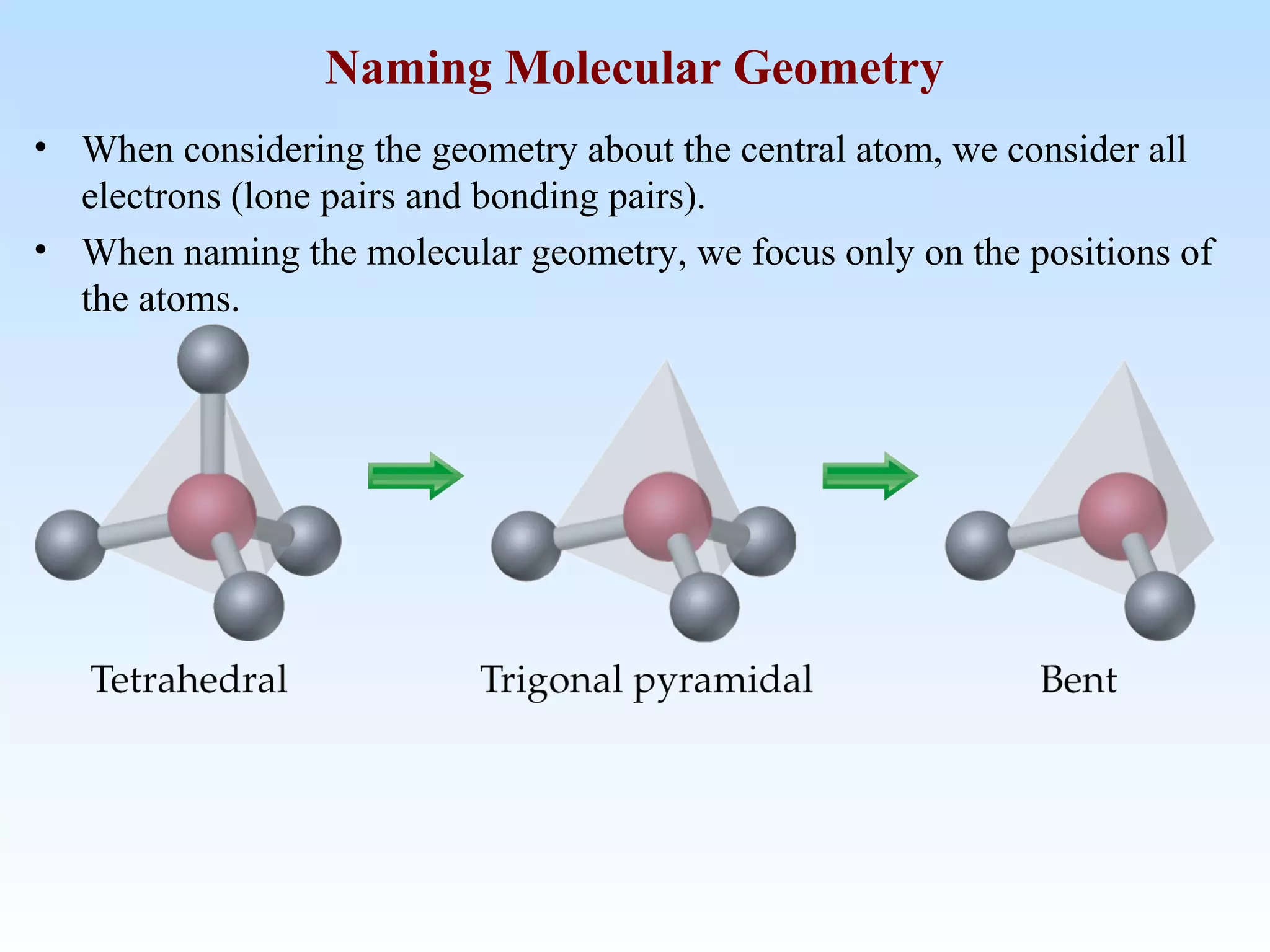
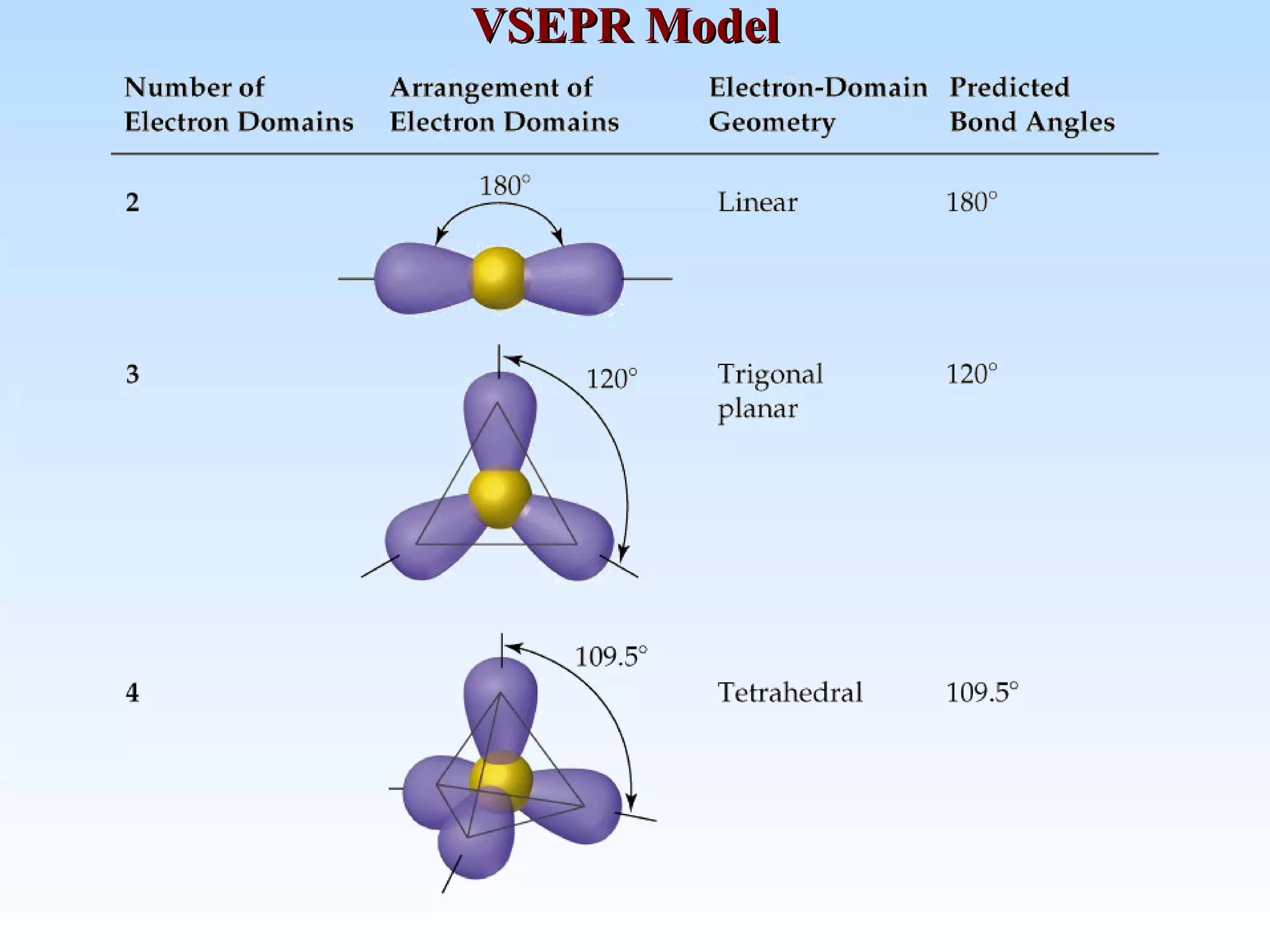
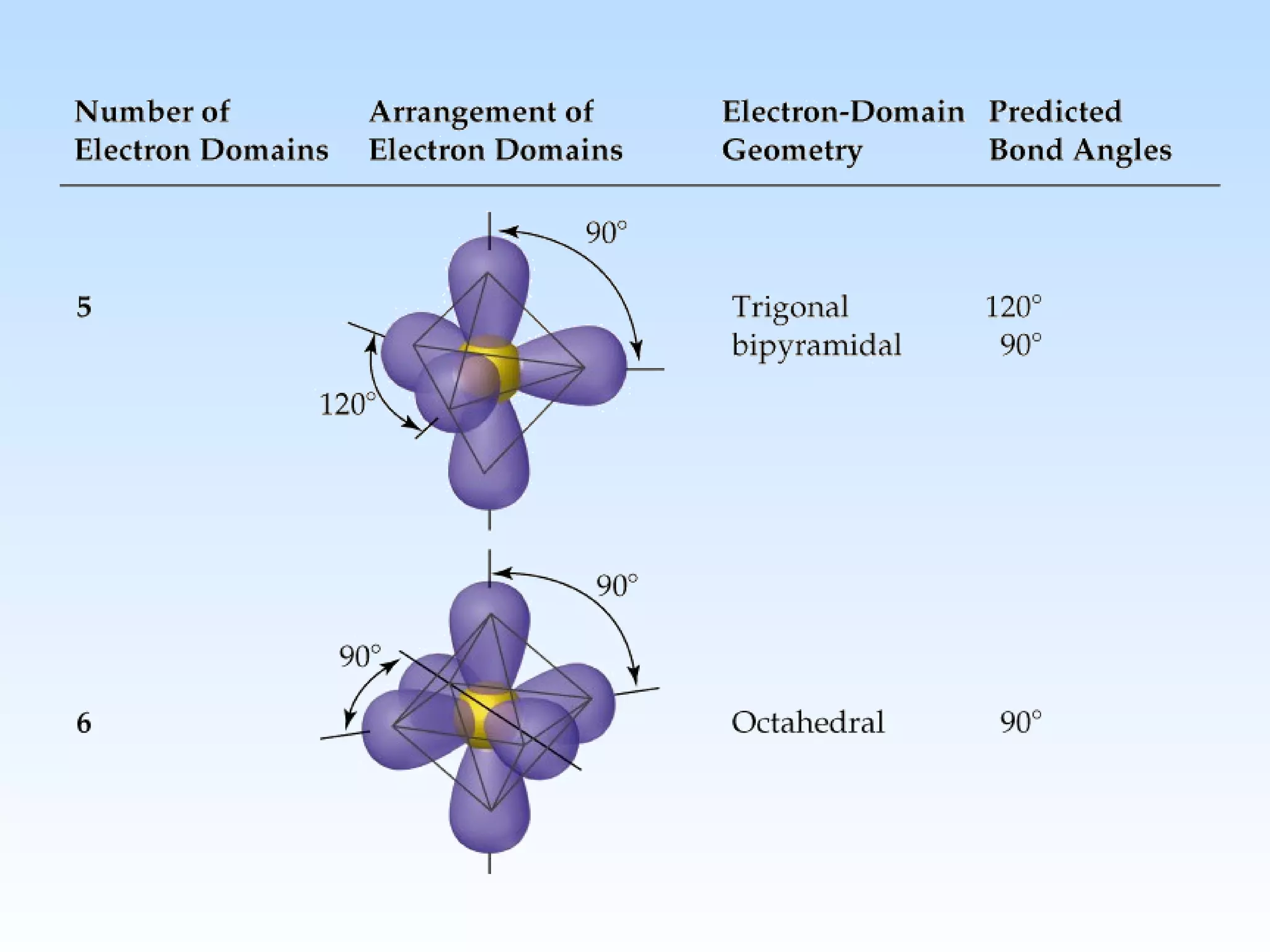
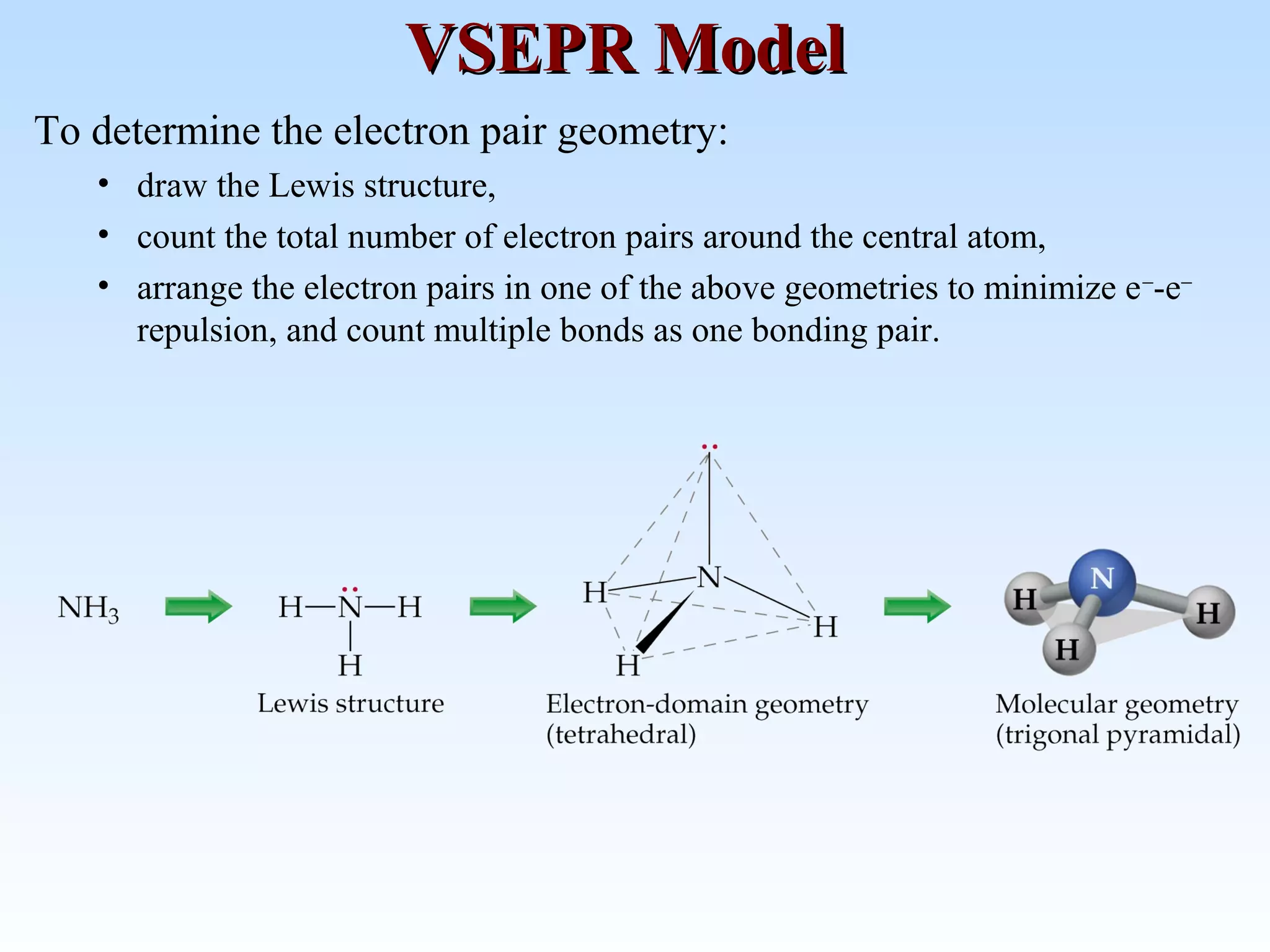
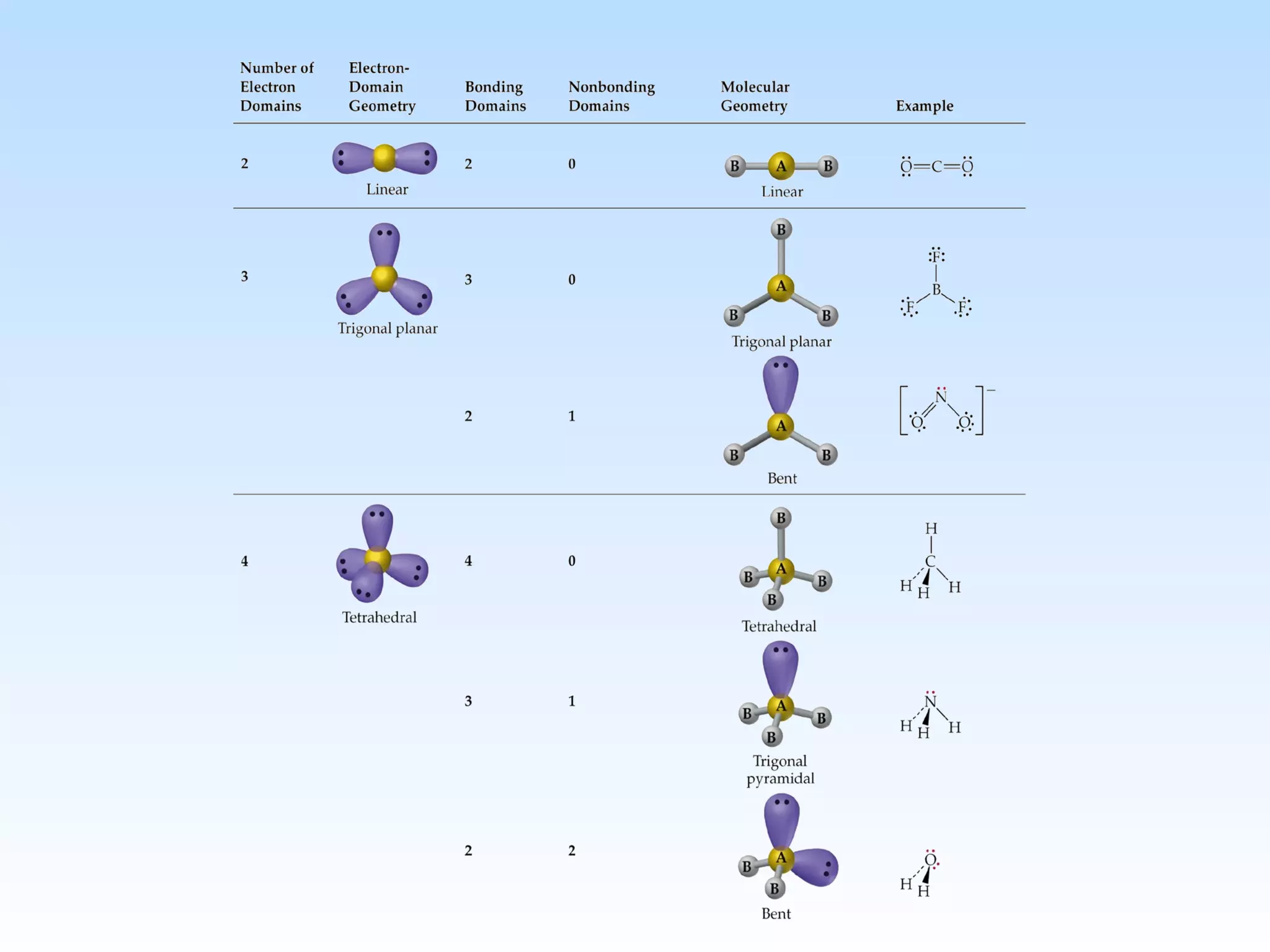
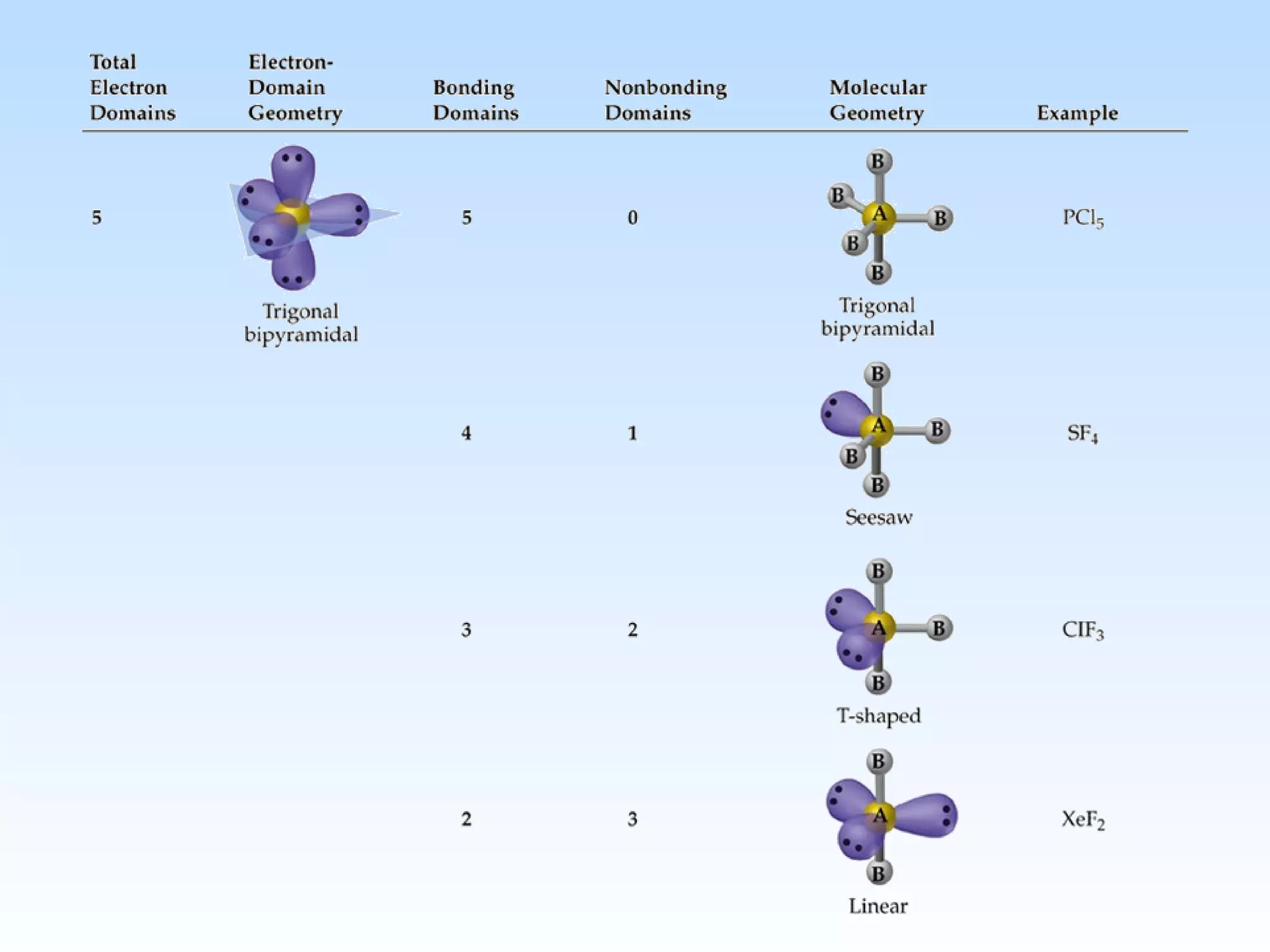
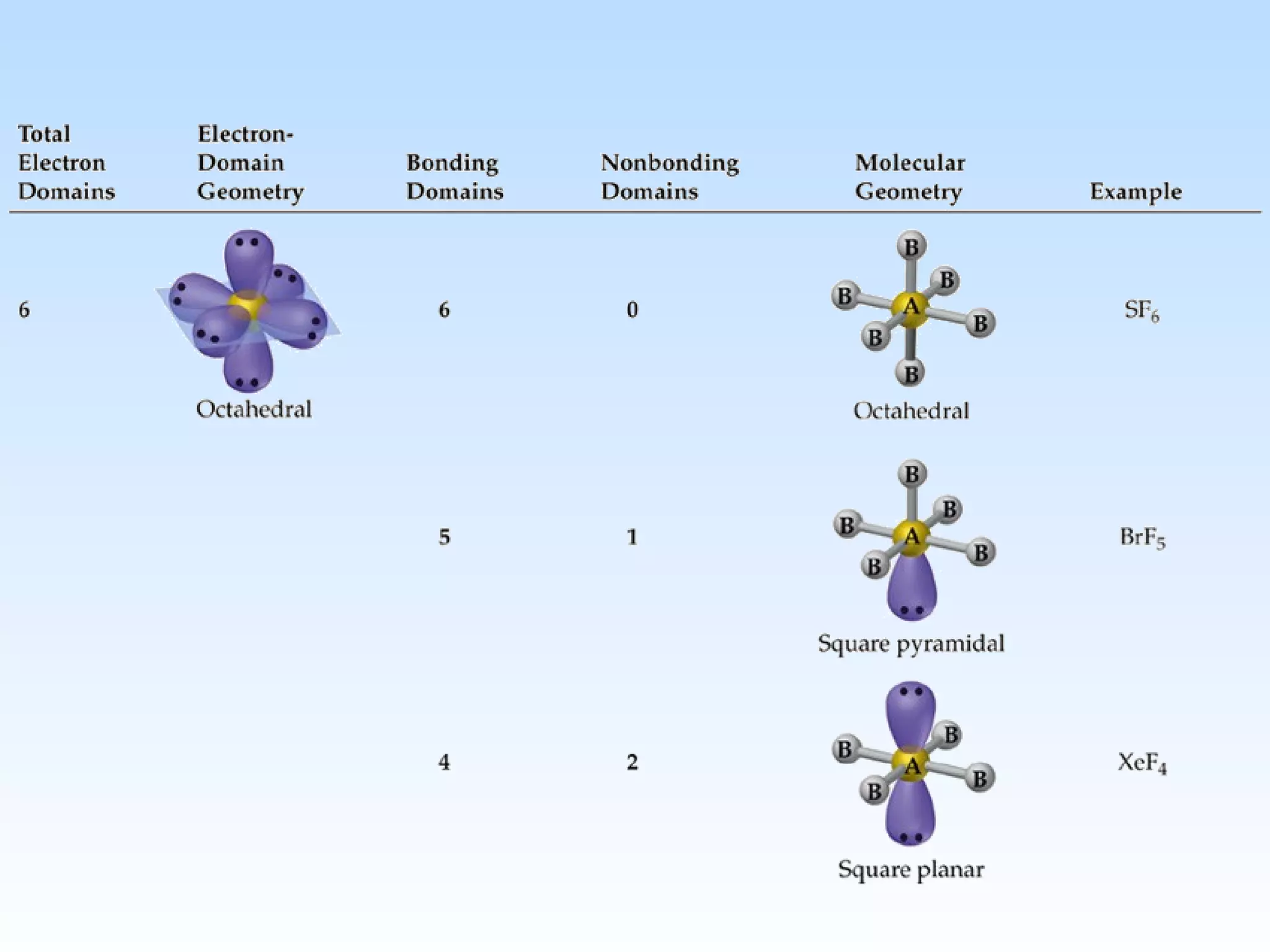
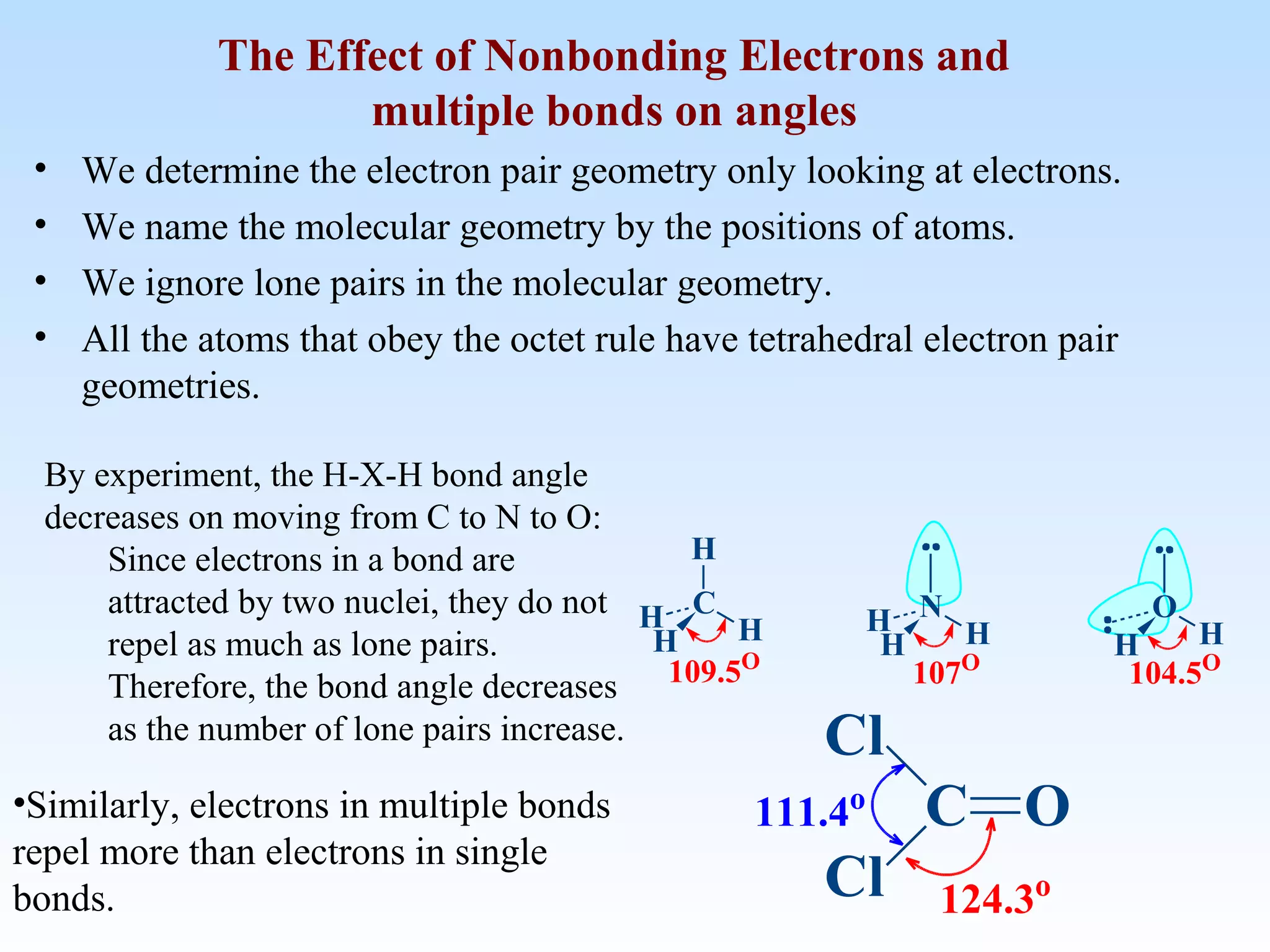
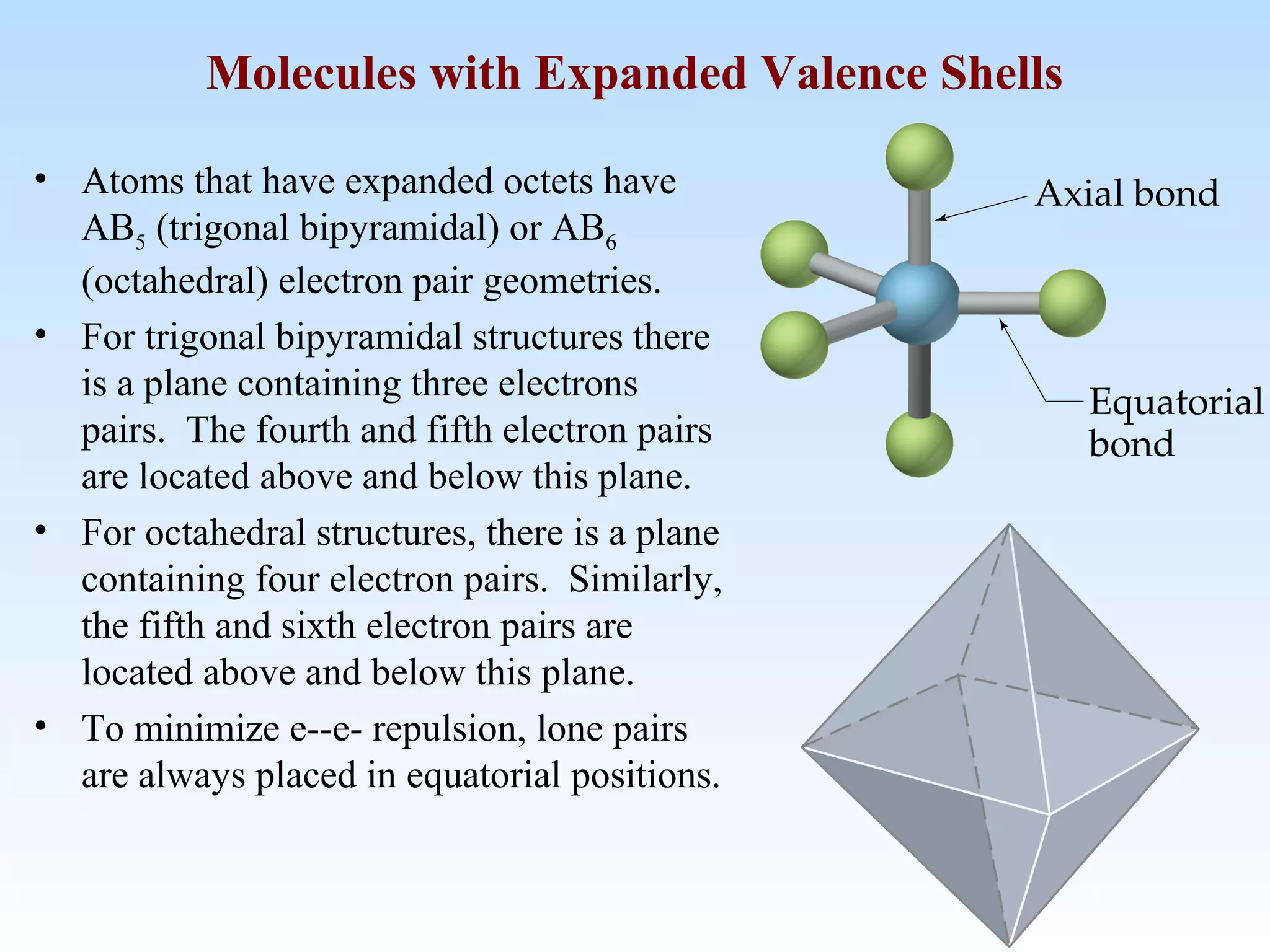
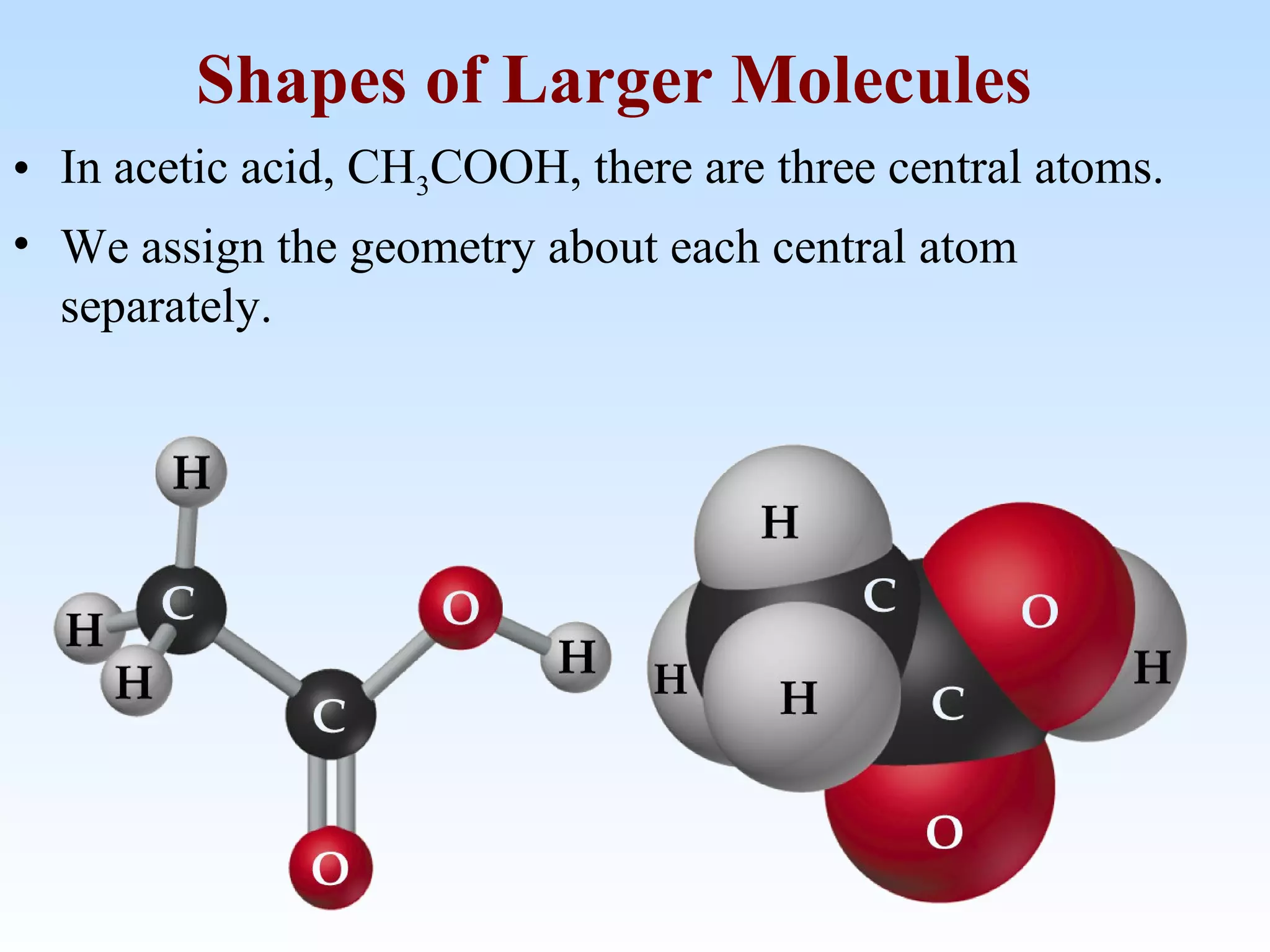
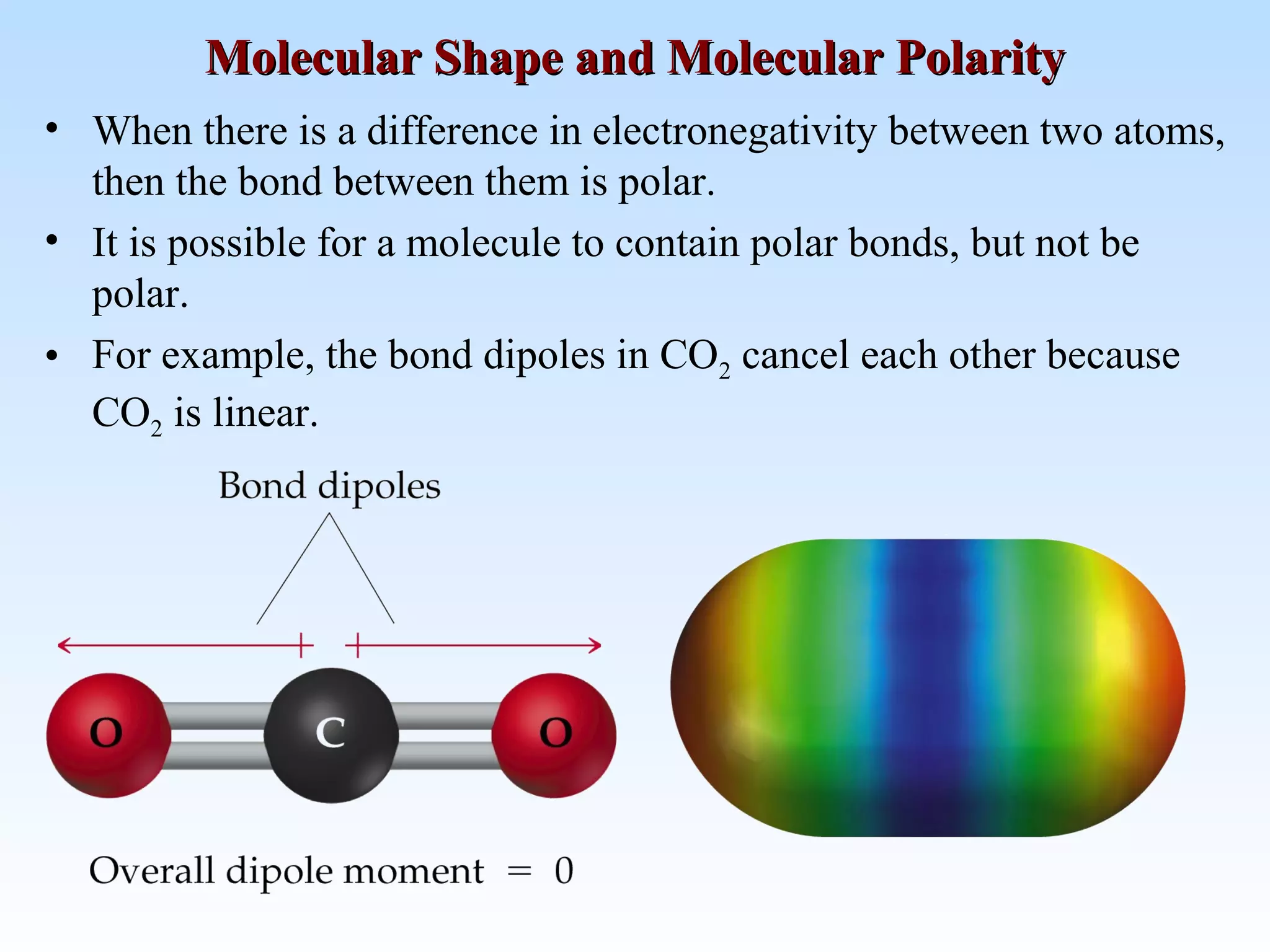
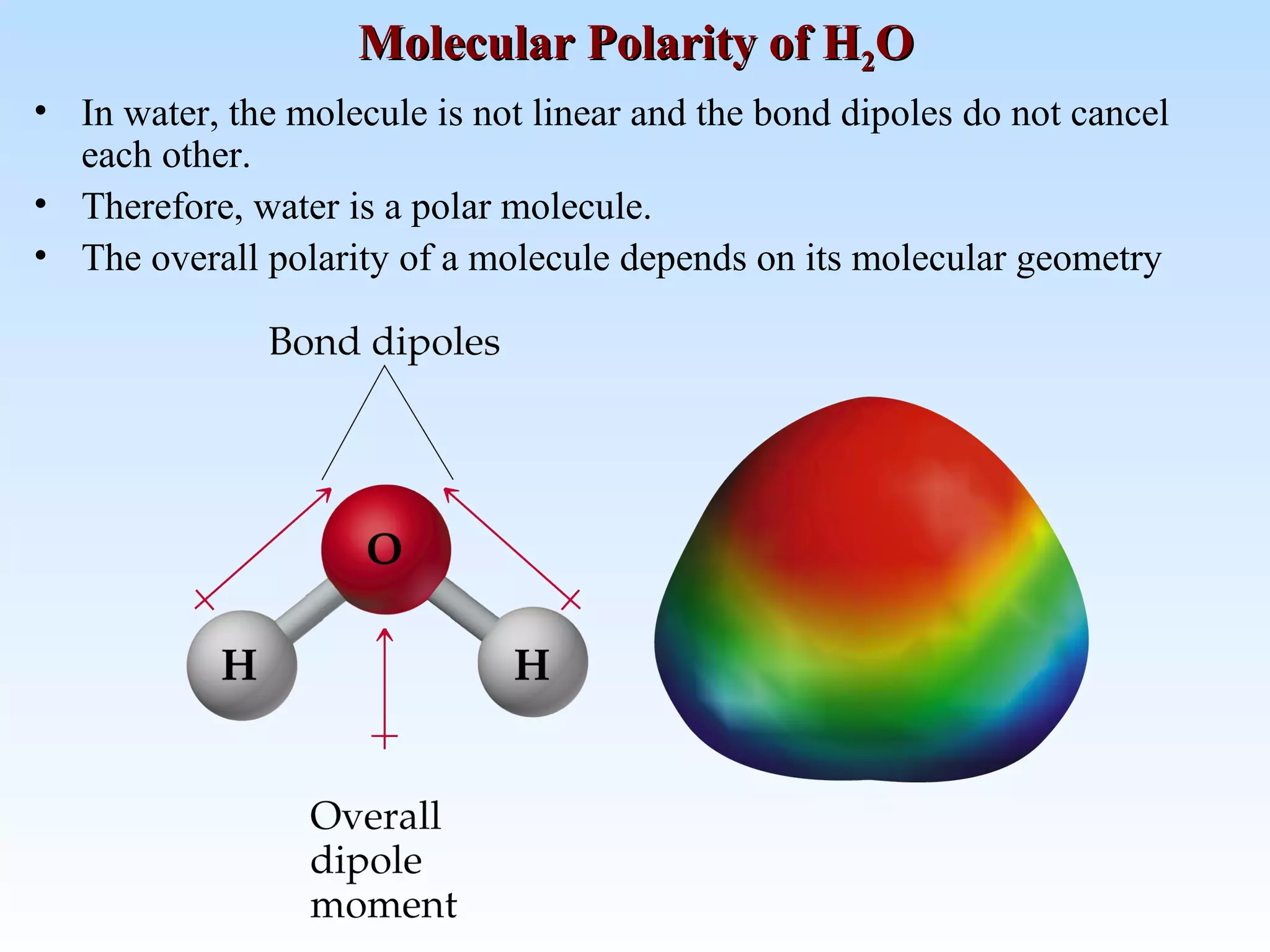
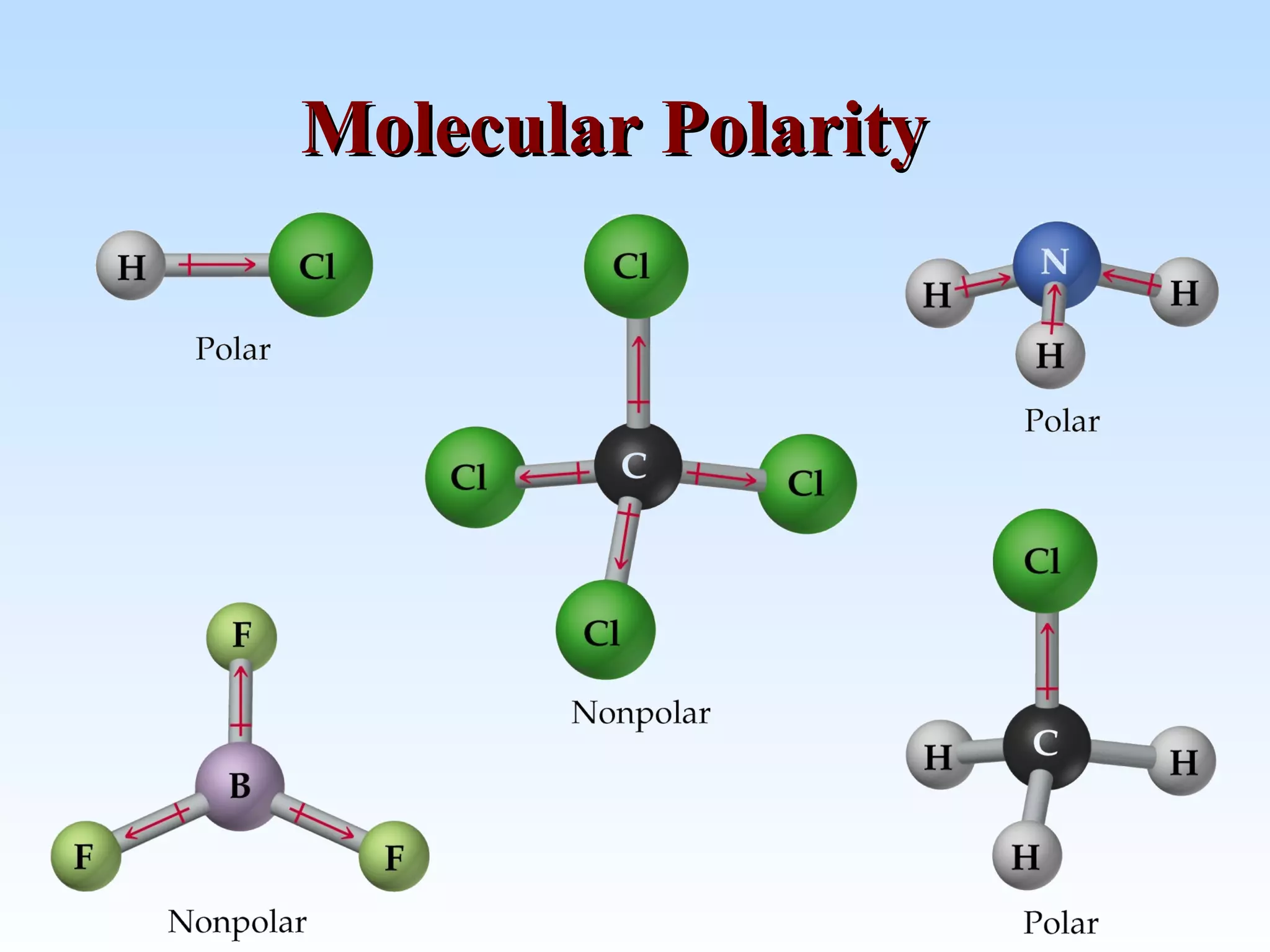
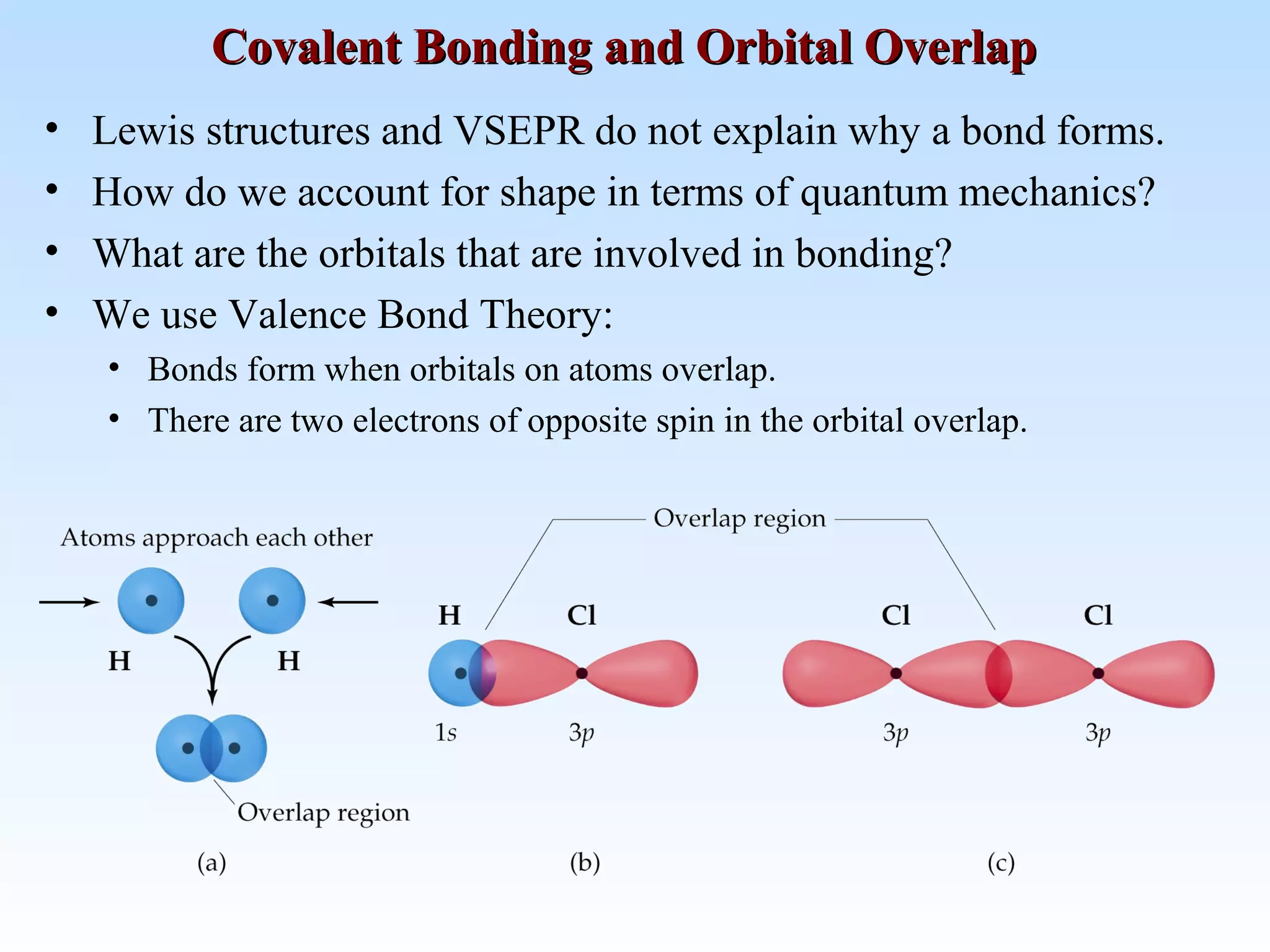
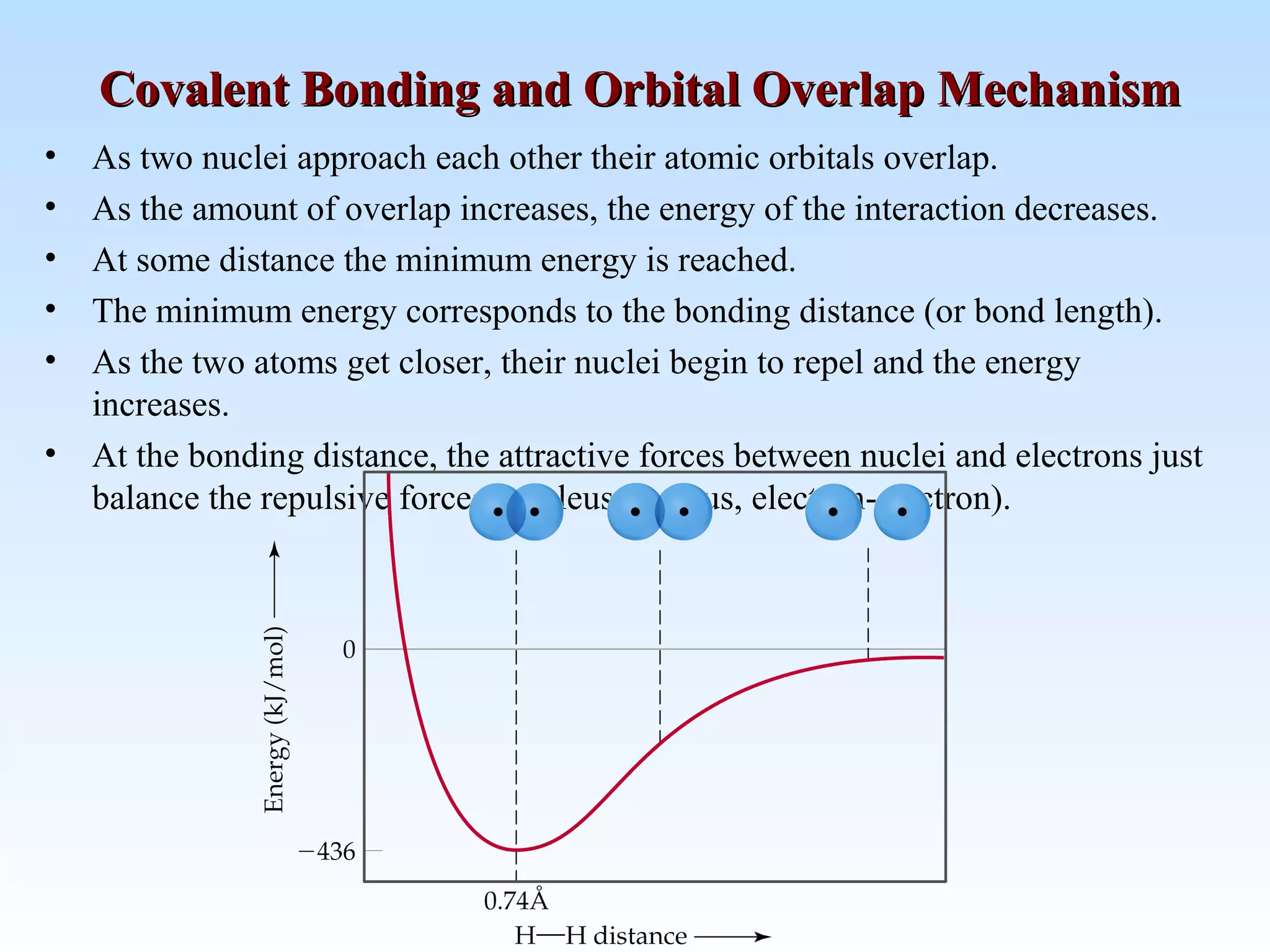
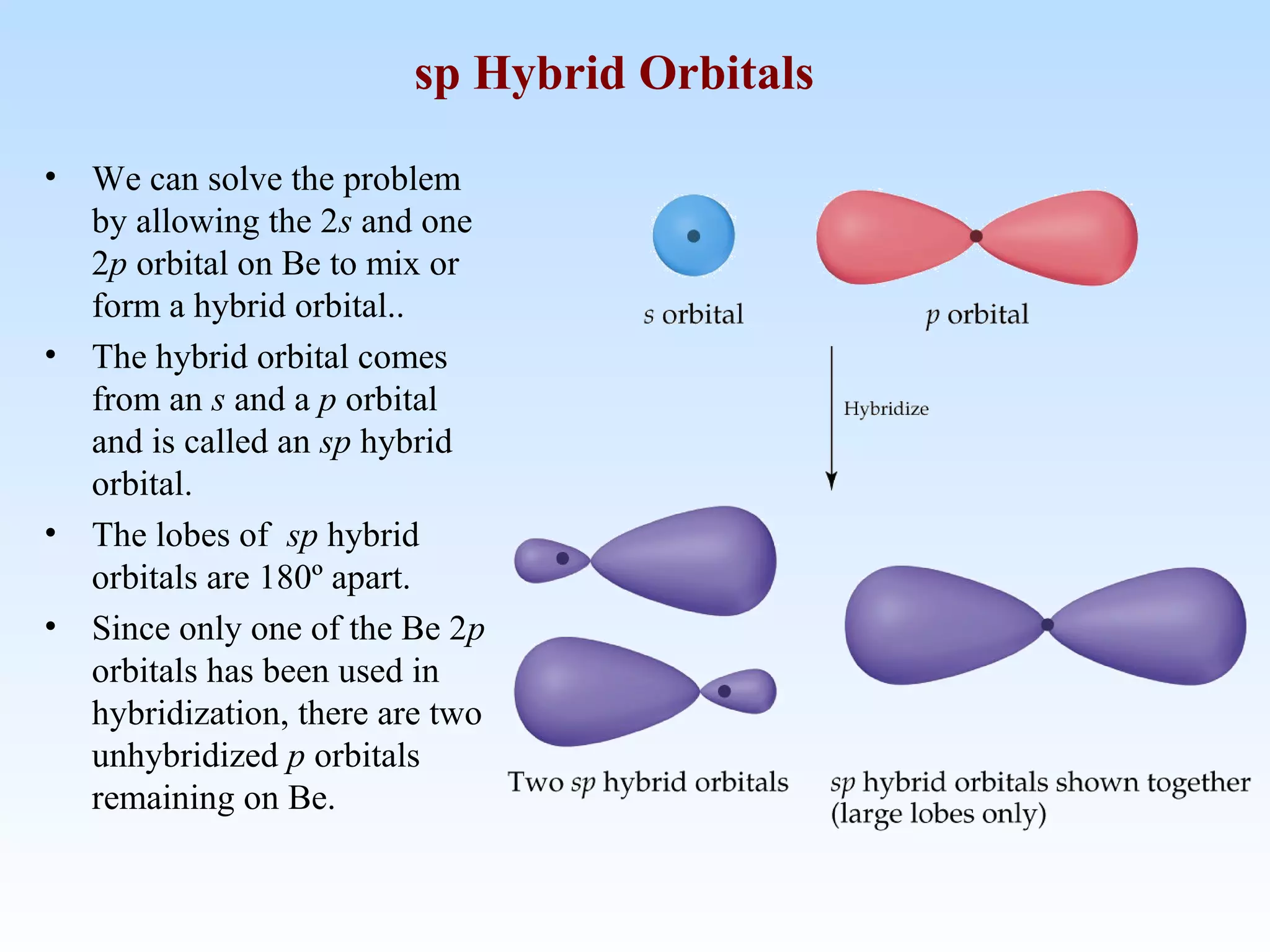
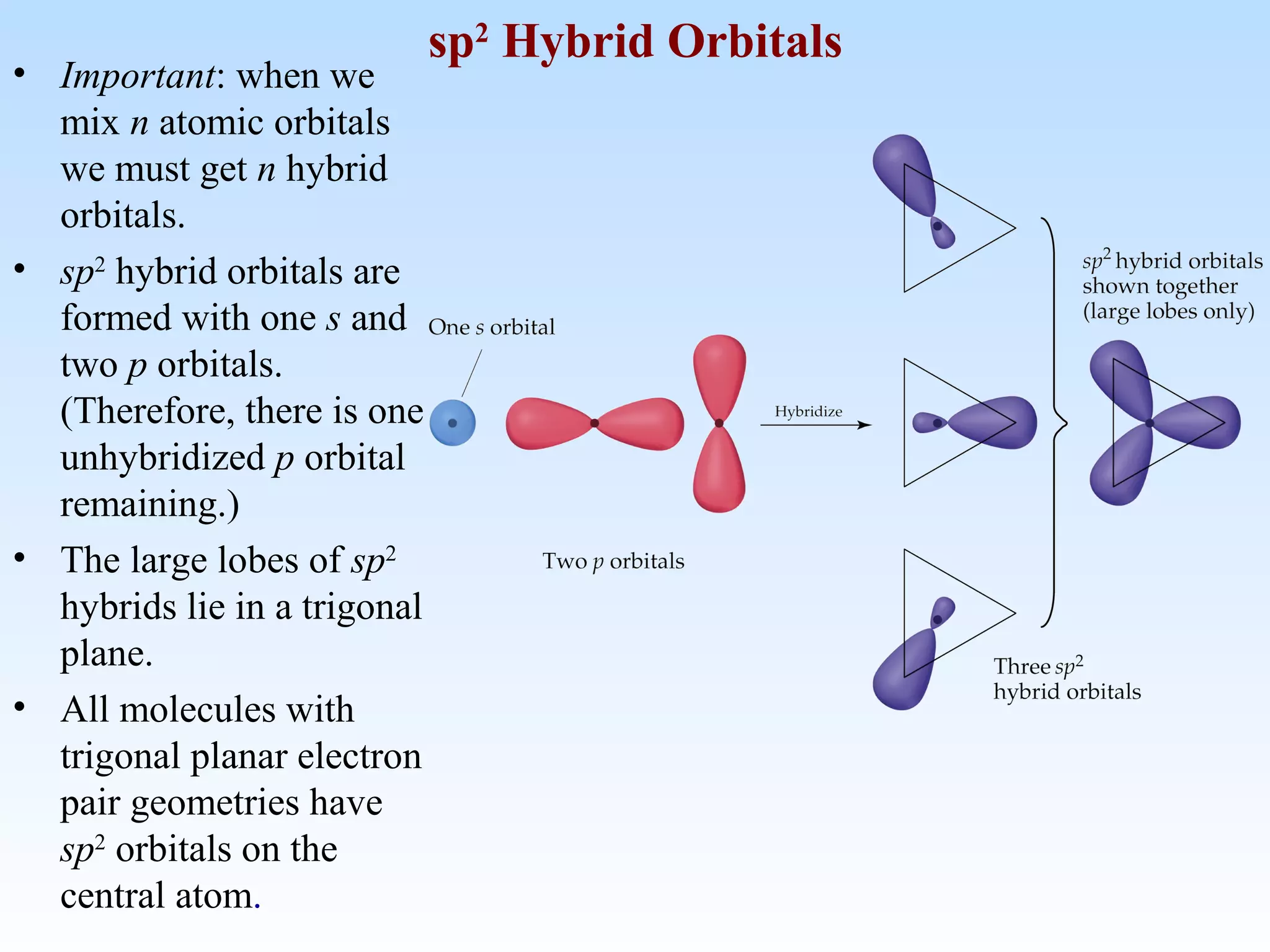
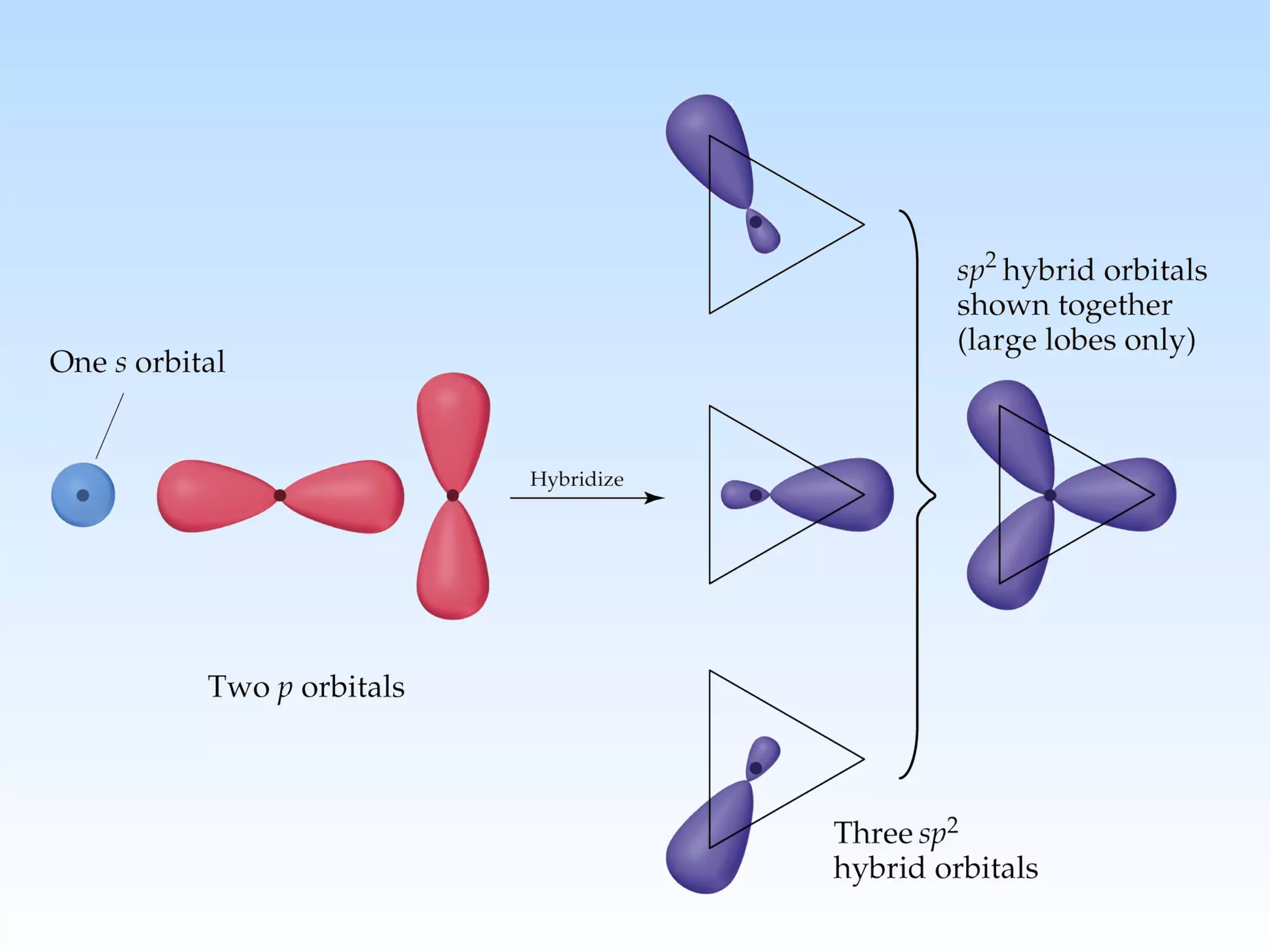
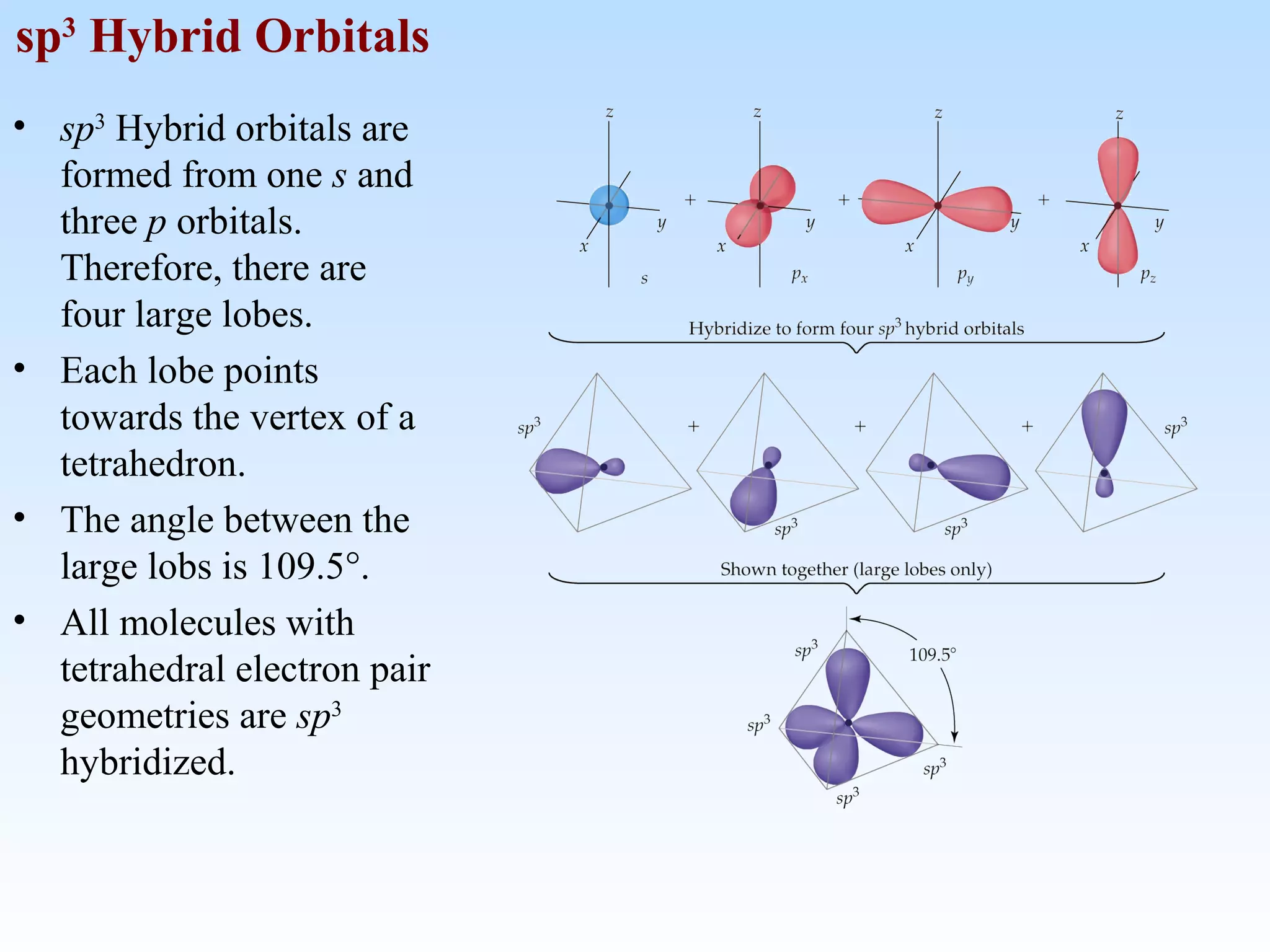
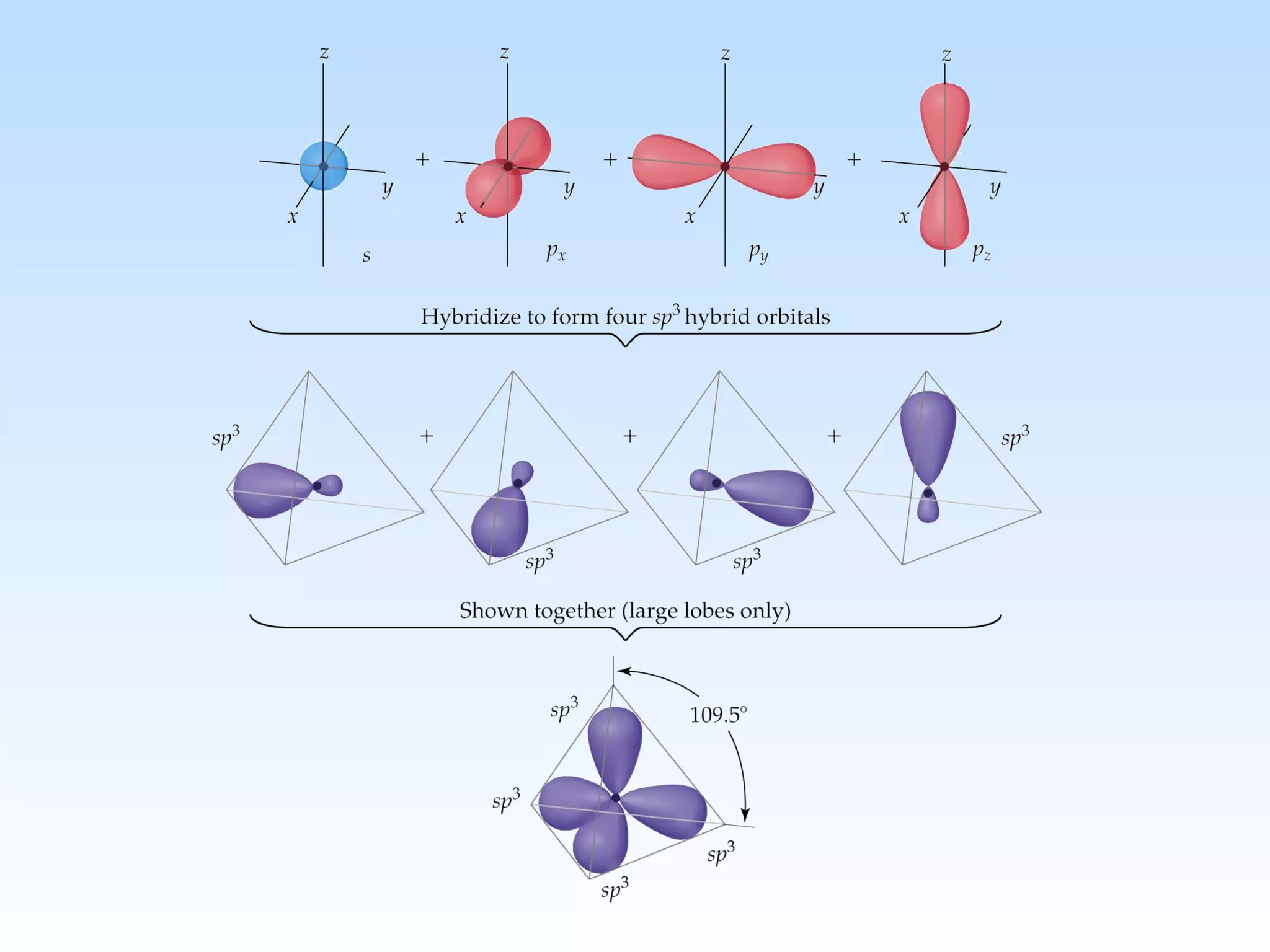
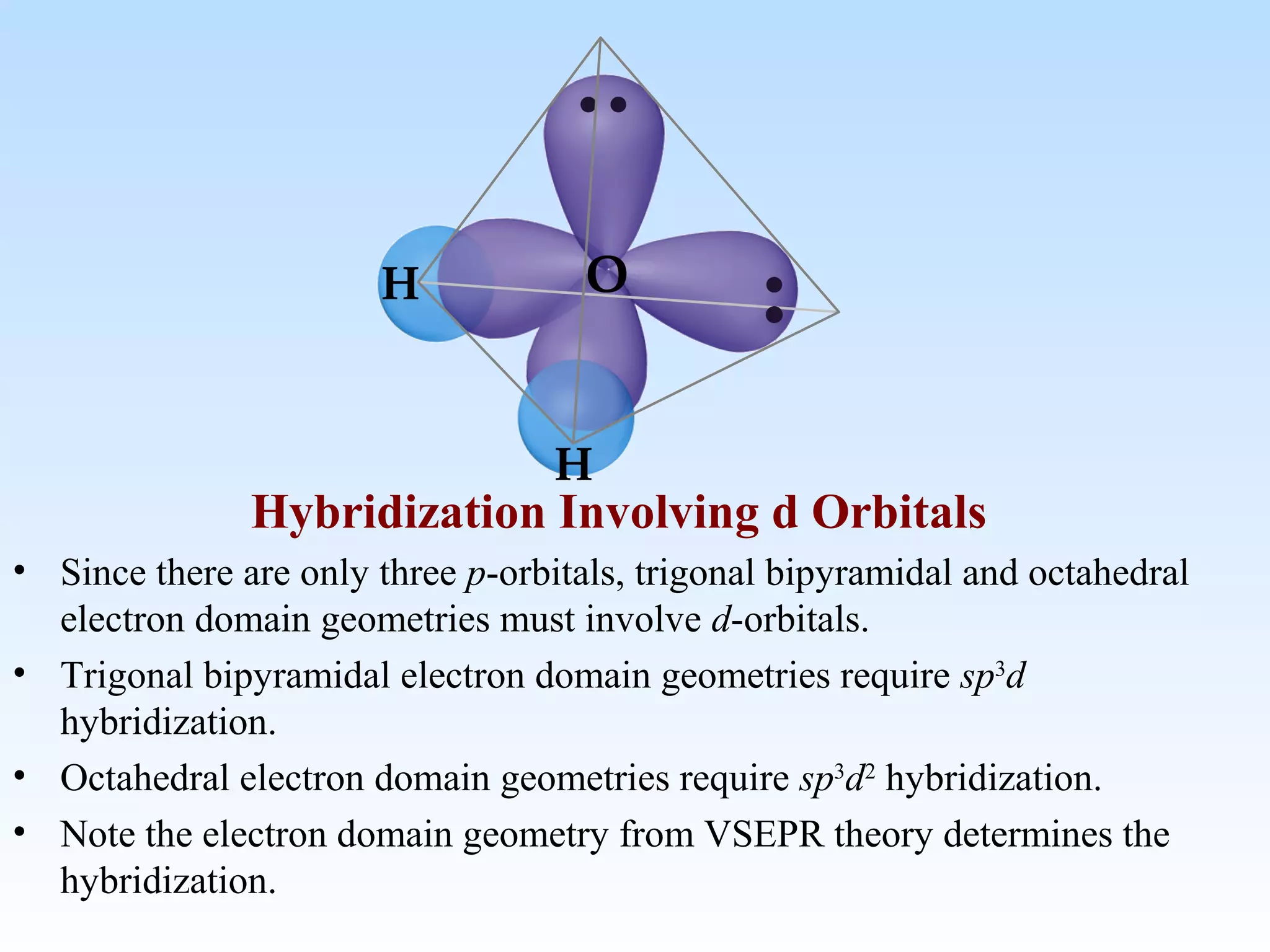
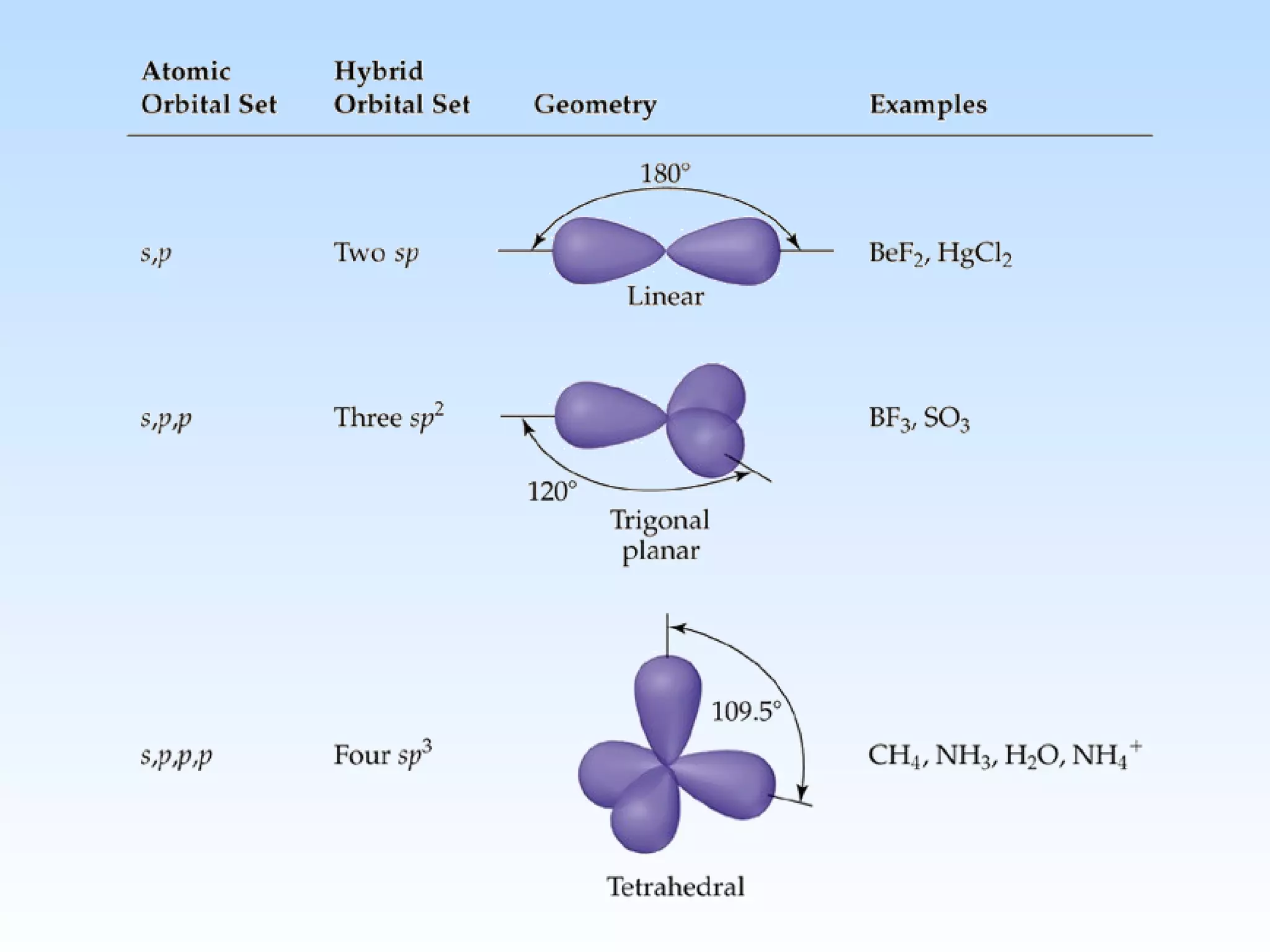
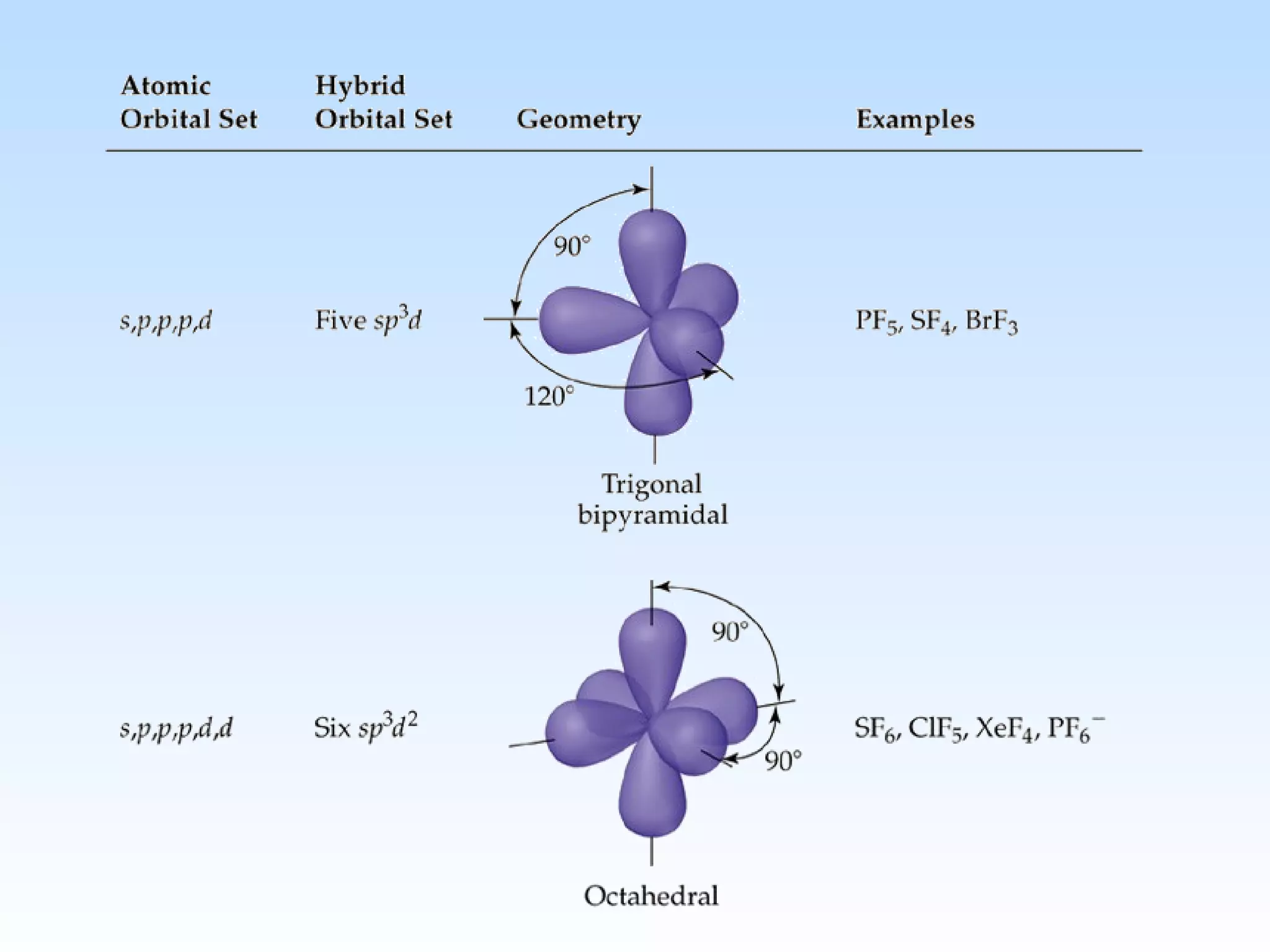
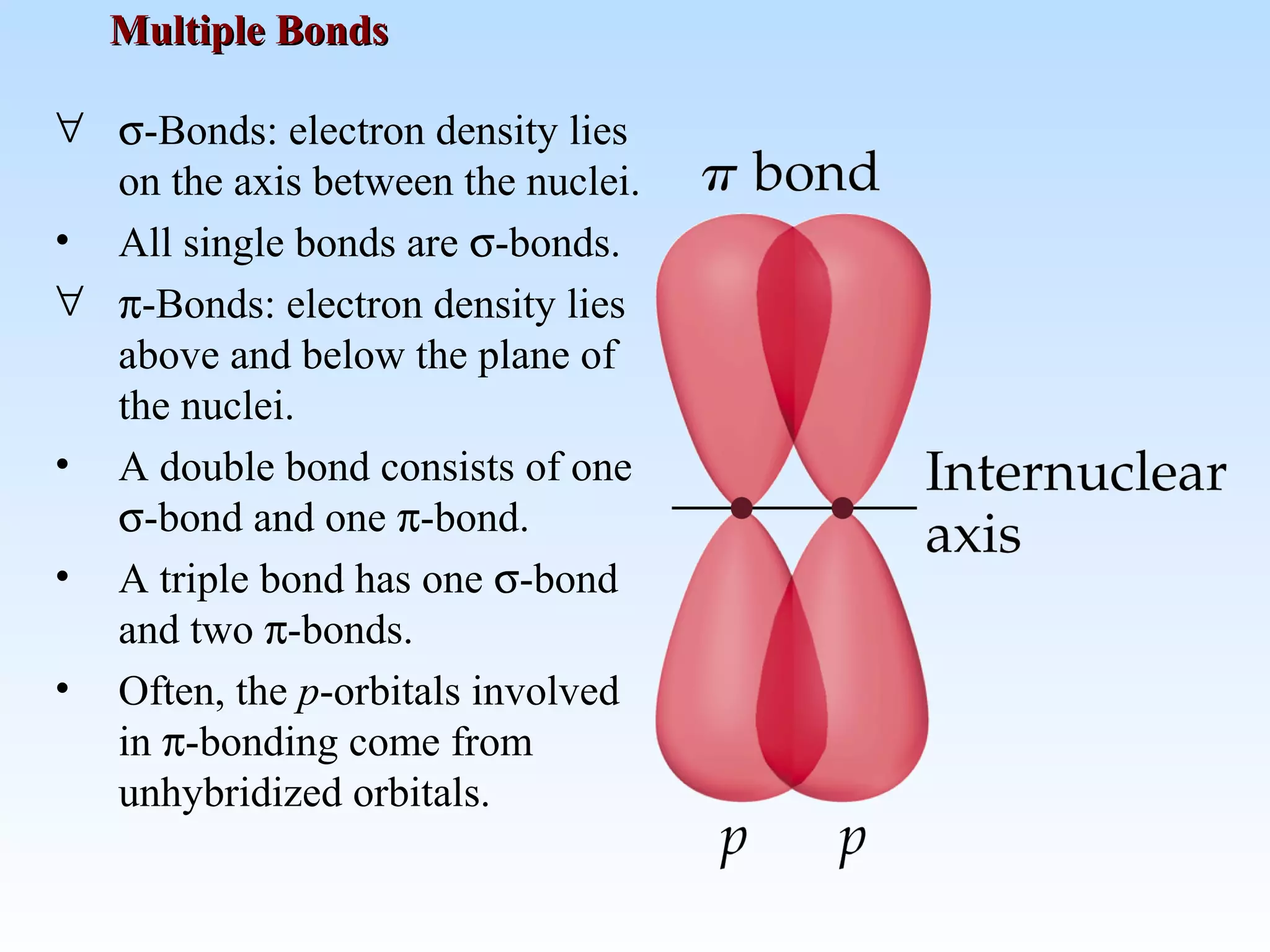
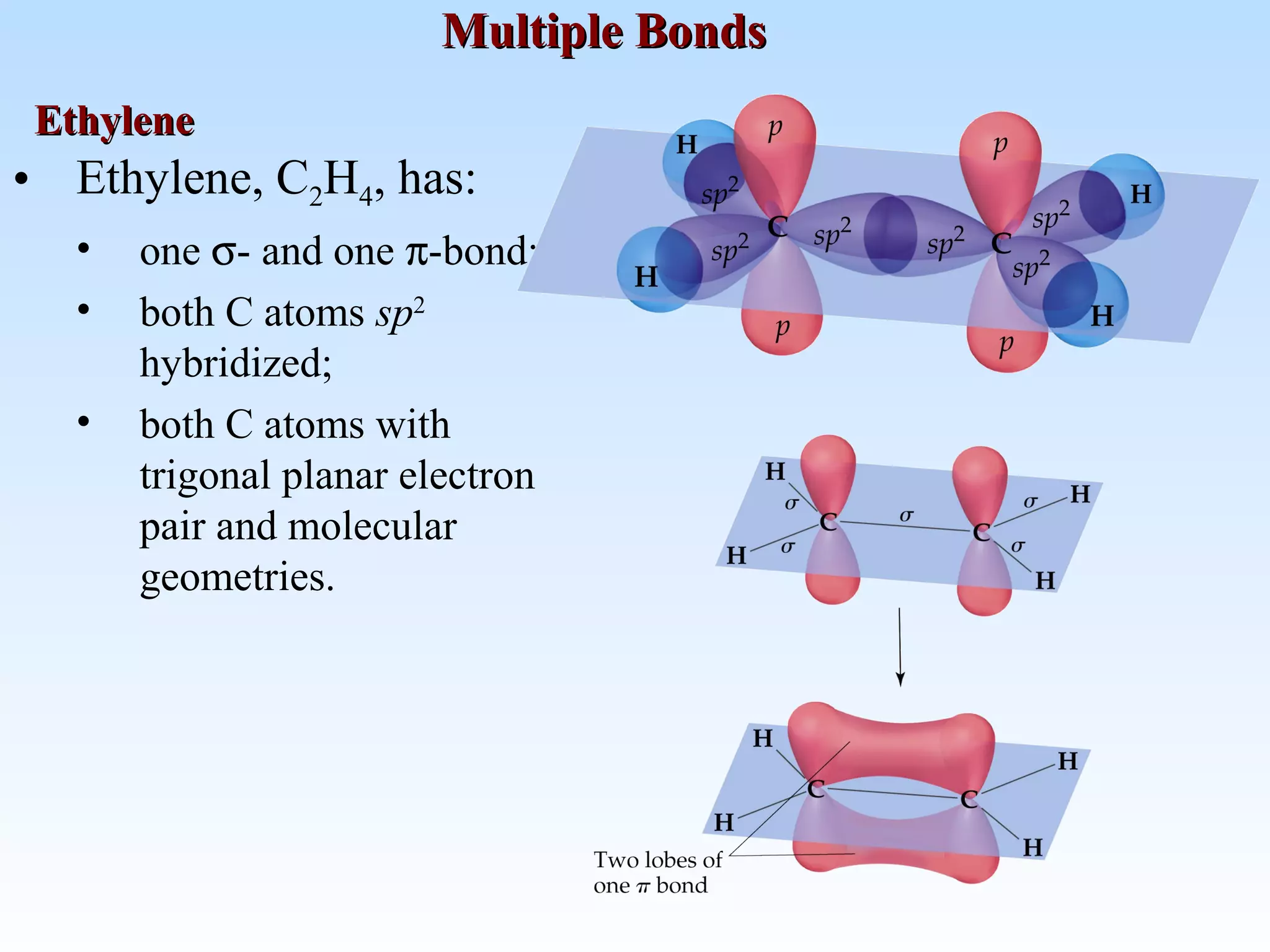
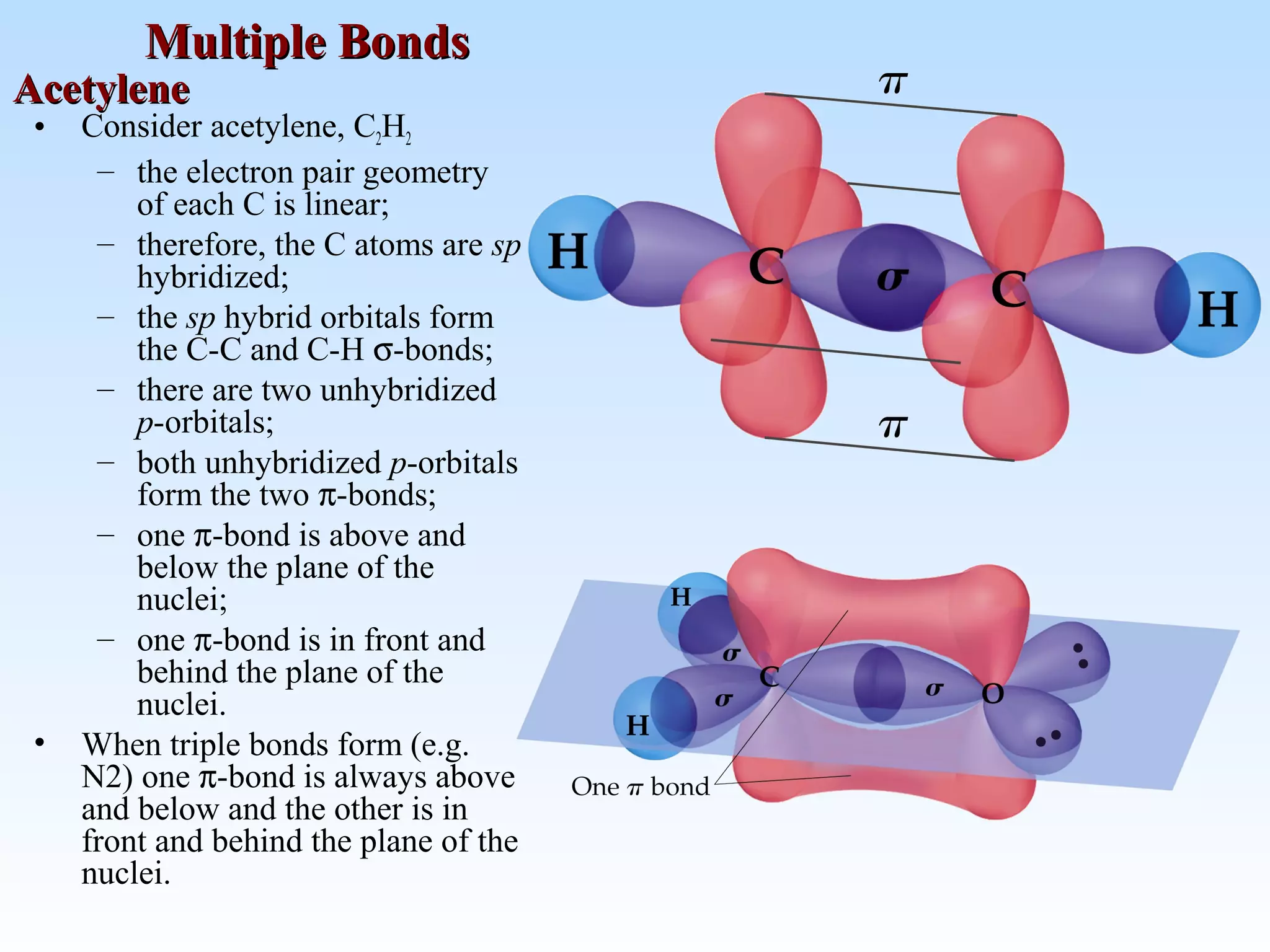
The document discusses molecular geometry and molecular orbitals, emphasizing that Lewis structures indicate atomic connectivity but not molecular shape, which is determined by bond angles. It introduces Valence Shell Electron Pair Repulsion (VSEPR) theory to predict molecular shapes based on electron pair repulsion and outlines hybridization concepts necessary for forming bonds. Additionally, it explains how molecular polarity arises from the geometry of molecules and the nature of bonding interactions, including sigma and pi bonds.