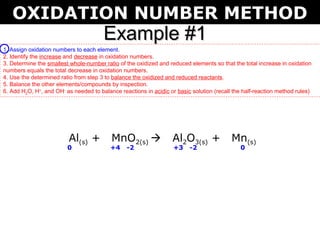
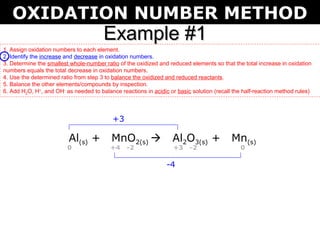
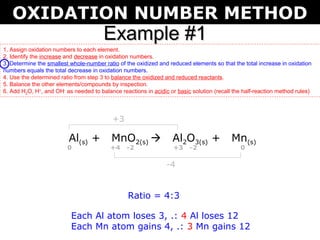
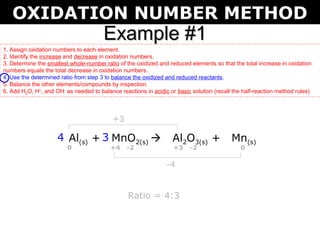
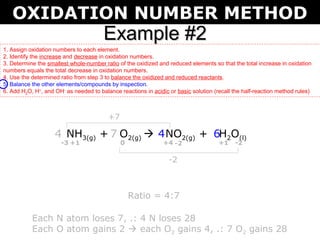
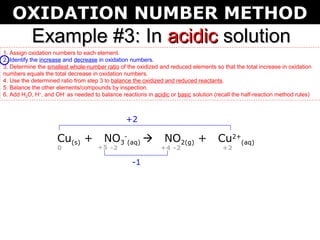
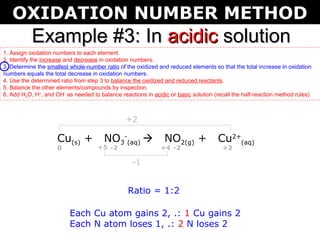
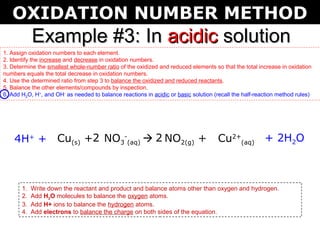
The document describes the oxidation number method for balancing redox reactions. It provides two examples showing the steps:
1) Assign oxidation numbers and identify changes
2) Determine the ratio that balances the changes
3) Use the ratio to balance the reactants and products
4) Balance other elements by inspection
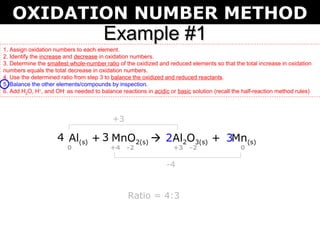
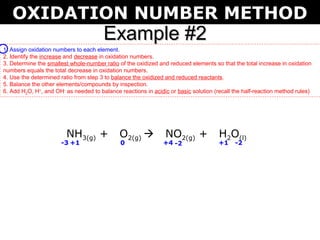
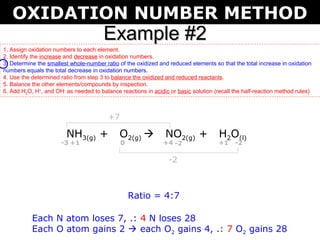
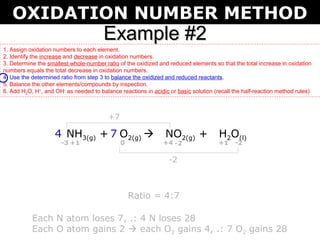
The first example balances the reaction of aluminum with manganese dioxide to form aluminum oxide and manganese. The second example balances the reaction of ammonia with oxygen to form nitrogen dioxide and water.