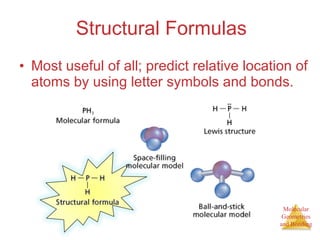
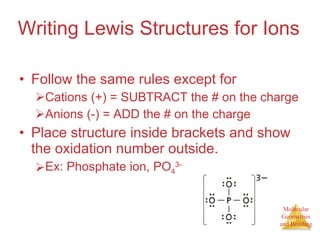
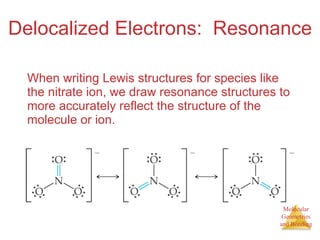
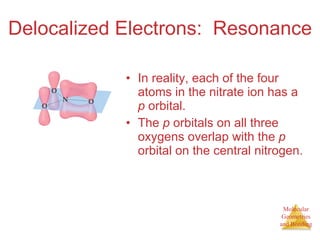
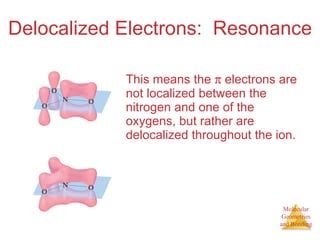
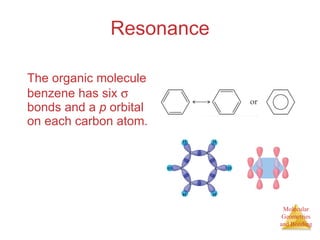
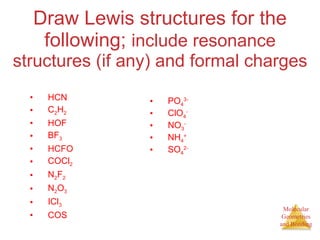
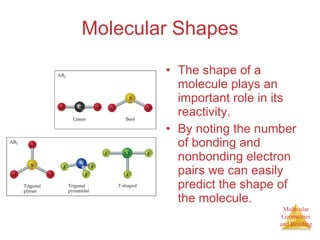
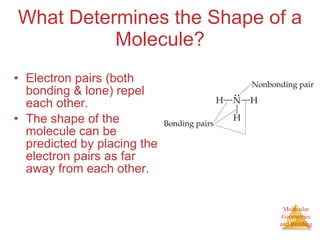
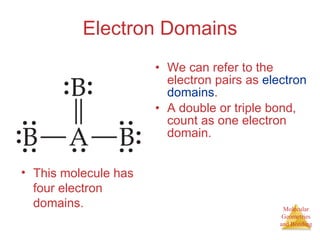
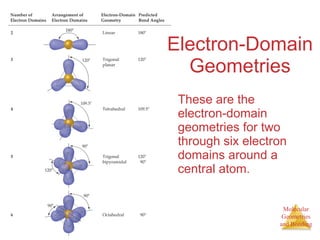
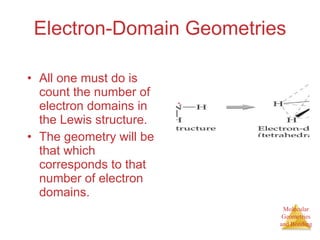
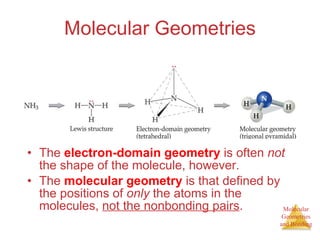
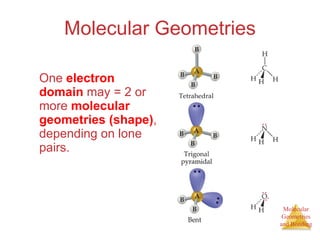
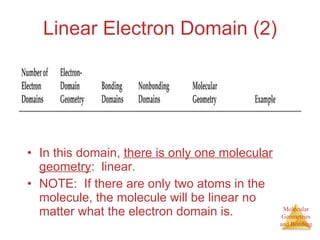
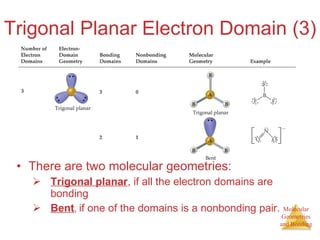
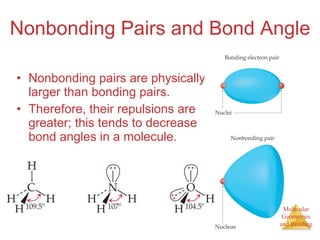
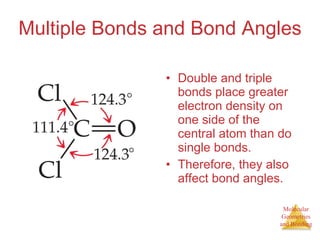
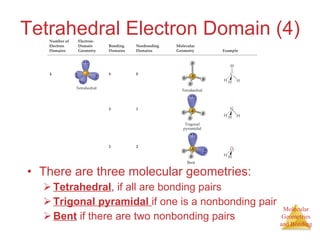
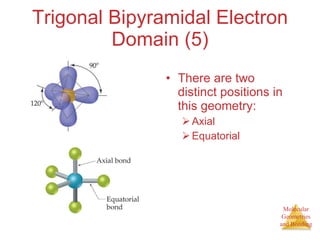
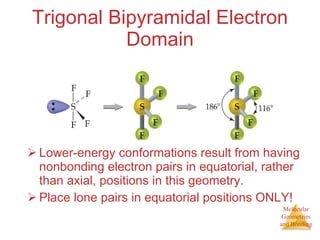
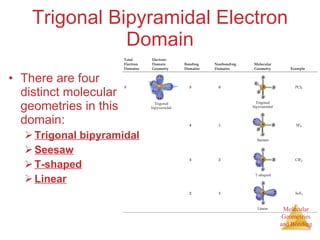
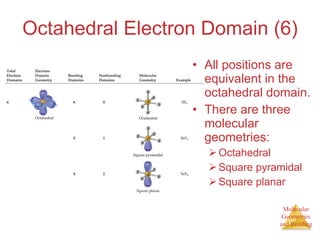
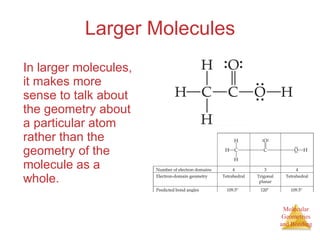
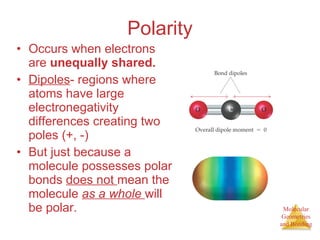
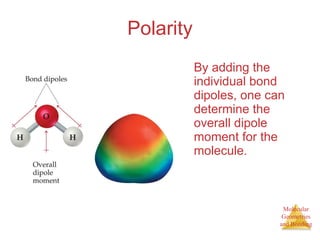
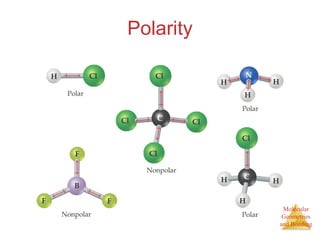
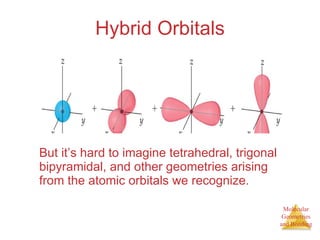
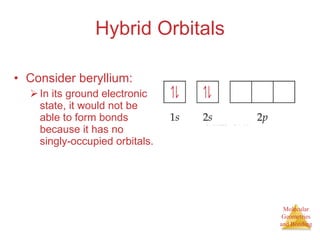
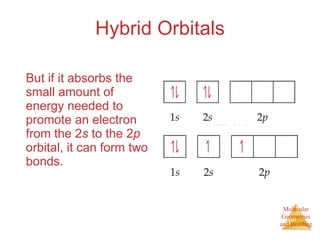
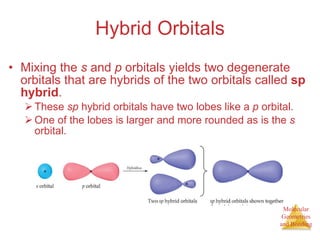
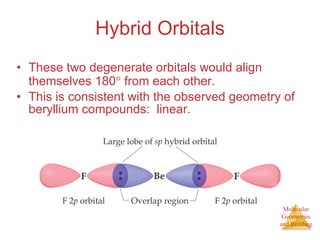
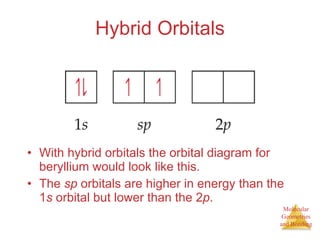
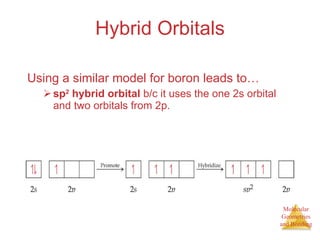
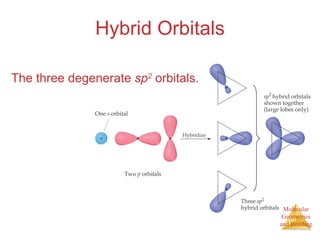
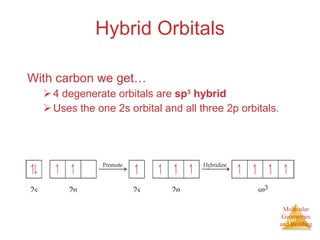
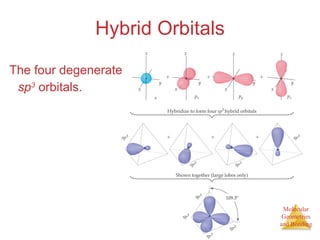
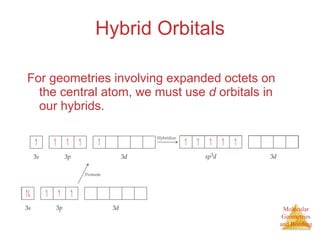
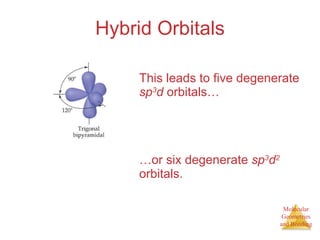
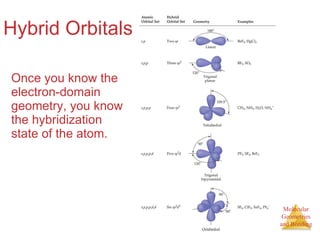
The document discusses molecular structures and resonance, molecular geometries and bonding theories, and electronegativity and polarity. It explains how to write Lewis structures, including showing resonance structures. It describes how molecular geometry can be predicted from the number of electron pairs around an atom. Valence shell electron pair repulsion theory is used to determine molecular shapes. Hybrid orbital theory is introduced to explain molecular geometries involving s, p, and d orbitals.