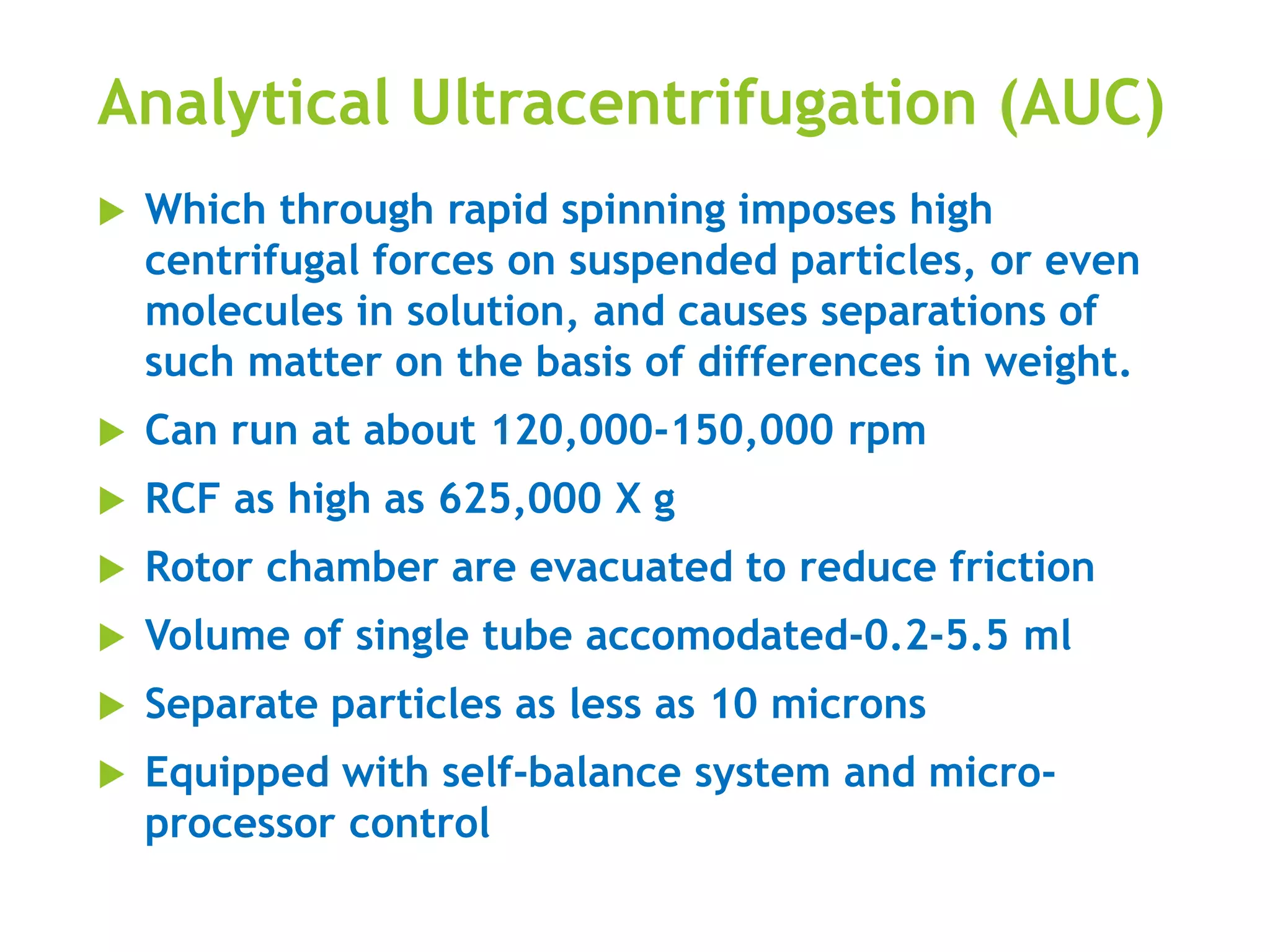
The document discusses analytical ultracentrifugation and centrifugation techniques used in various scientific fields for the separation of heterogeneous mixtures. It details different types of centrifuge rotors, their functionalities, and explains processes like sedimentation velocity experiments and sedimentation equilibrium experiments. Additionally, it highlights the significance of marker enzymes in isolating specific organelles during cell fractionation.