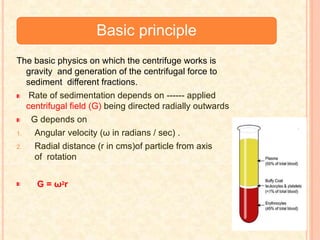
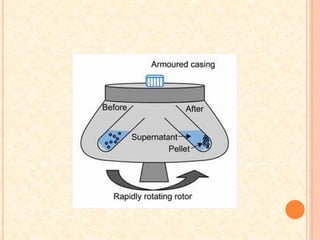
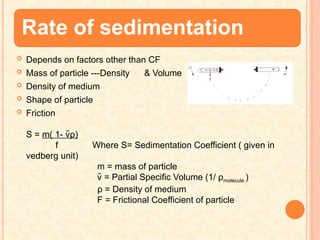
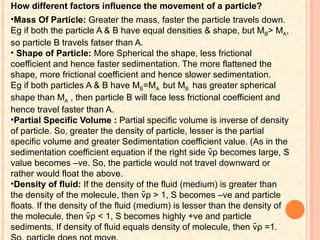
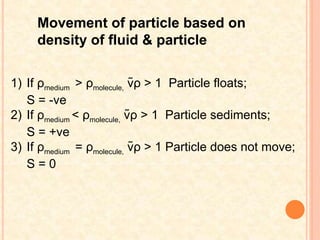
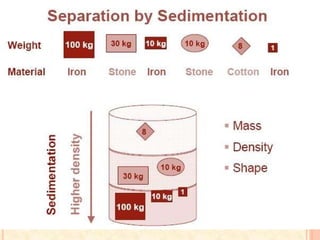
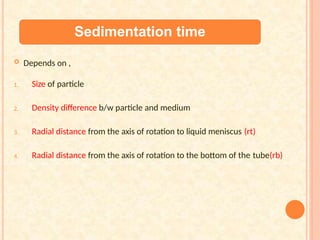
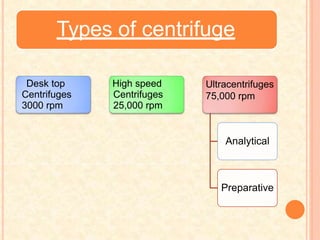
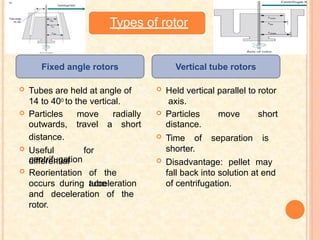
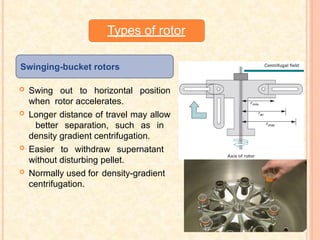
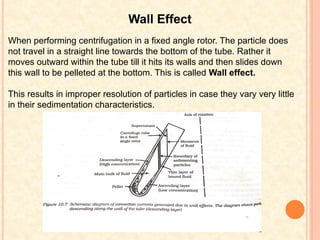
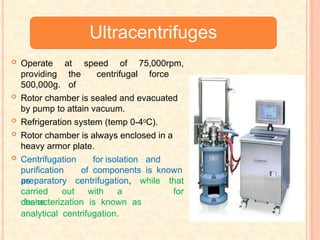
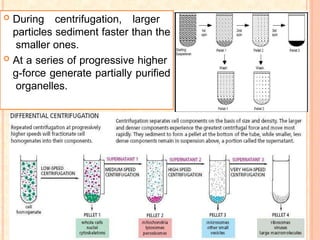
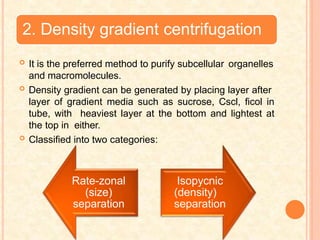
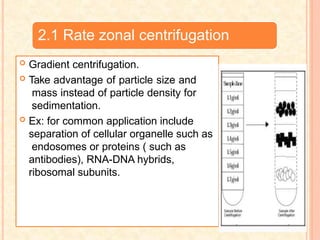
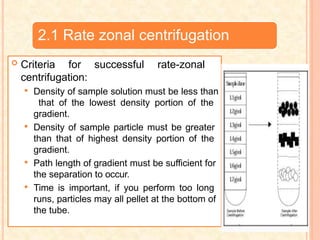
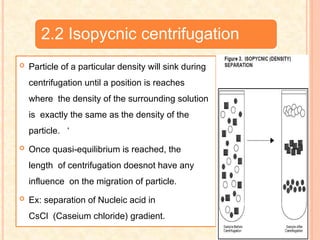
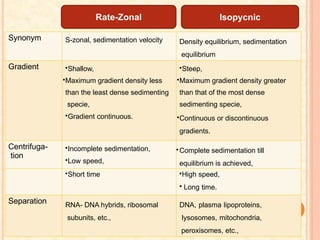
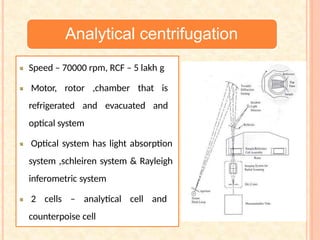
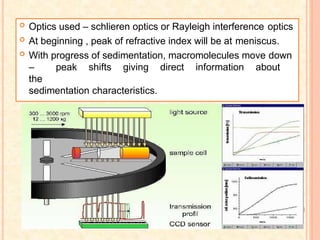
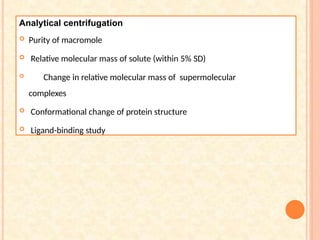
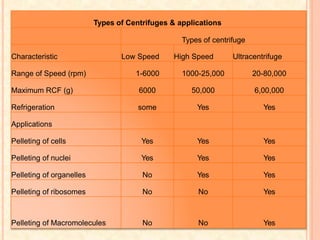
The document discusses the principles and applications of biological centrifugation, a technique used to separate and purify biological particles in liquid mediums based on centrifugal forces. It covers various types of centrifuges, their technical specifications, operational details, and factors influencing sedimentation rates, as well as methods such as differential and density gradient centrifugation for isolating biological materials. Furthermore, it highlights the importance of centrifugation in clinical laboratories for analyzing biological samples.