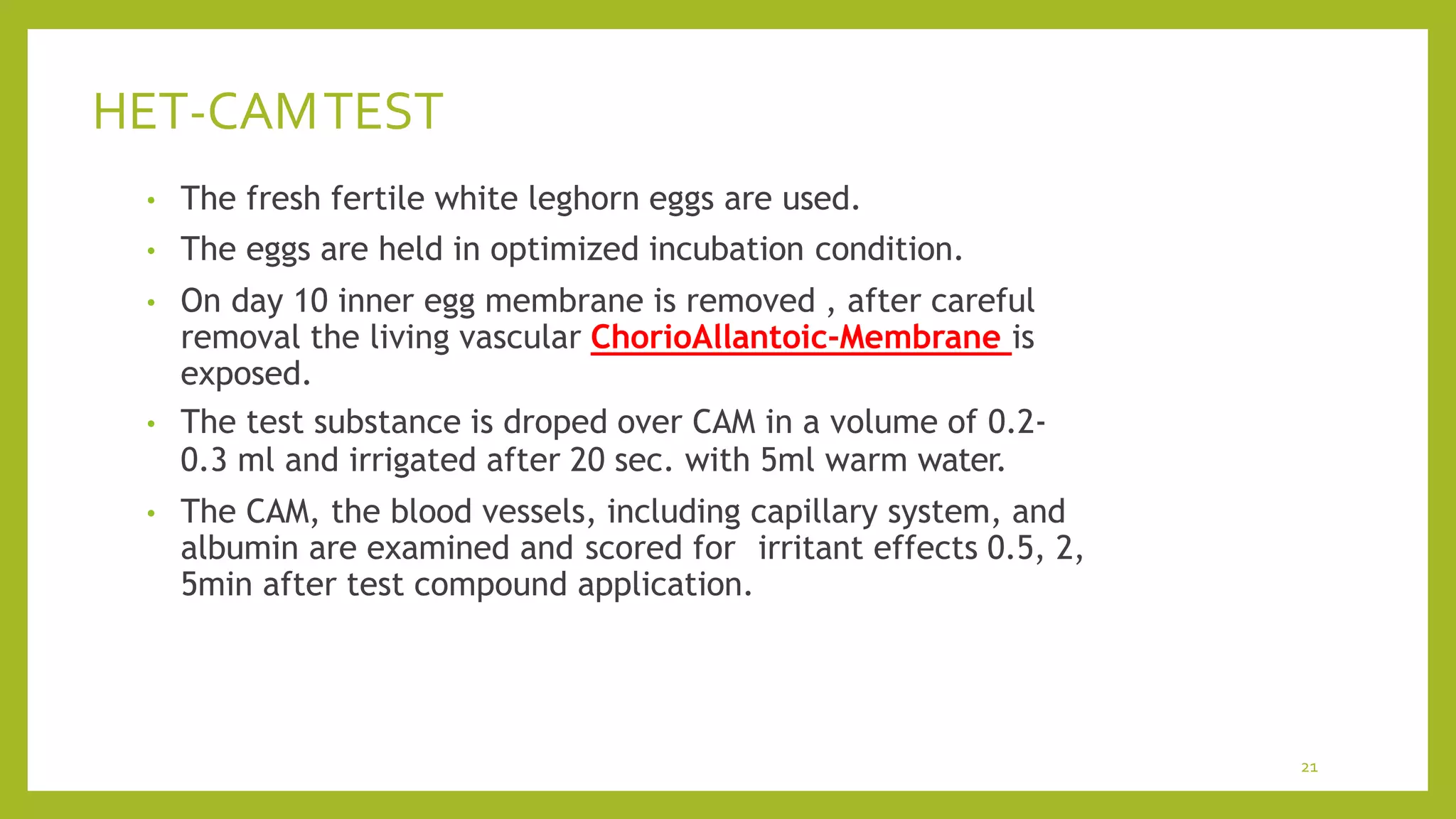
The document discusses alternative methods to animal toxicity testing, emphasizing the '3Rs' principle: reduce, refine, and replace, alongside the need for humane and effective research techniques. It highlights various in vitro and in silico methods, such as cell culture and computer modeling, that can minimize animal use while maintaining scientific integrity. Additionally, it details specific testing methods, including the HET-CAM test, designed to assess irritant effects using non-animal techniques.





![ALTERNATIVETOANIMAL EXPERIMENTS
Continued but modified use of animals 3Rs
In vitro[test tube] method
Tissue culture technique
In silico [computer modellingtechnique]
Computer aided molecular drugdesign[CADD]
Computer assisted learning[CAL]
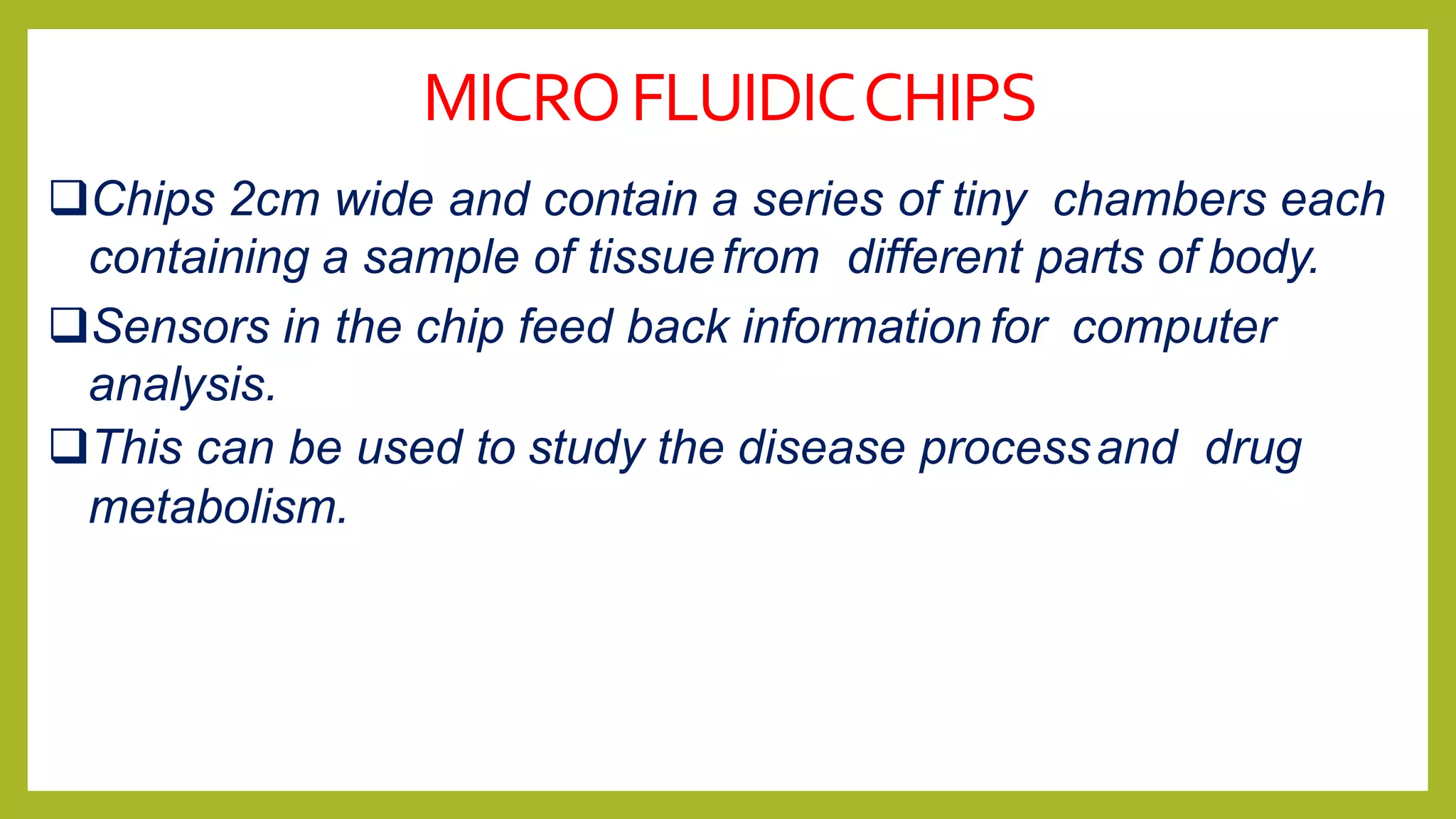
Microfluidic chips
Quantitative structure activity relationship
Organ on chips
Computer or mathematicalanalysis](https://image.slidesharecdn.com/alternativemethodstoanimaltoxicitytestingbysanchitdhankhar-210612041919/75/Alternative-methods-to-animal-toxicity-testing-7-2048.jpg)
![Methods of refinement
Modified to reduce pain and distress inanimal
Providing relief [pain and distress]bygiving
drugs like analgesics, anesthetics,
tranquillizers and sedatives.
By changing procedure:
Use non invasive technique like MRI
Use less sensitive animal species
Use smaller dose
Improving housing conditions](https://image.slidesharecdn.com/alternativemethodstoanimaltoxicitytestingbysanchitdhankhar-210612041919/75/Alternative-methods-to-animal-toxicity-testing-9-2048.jpg)

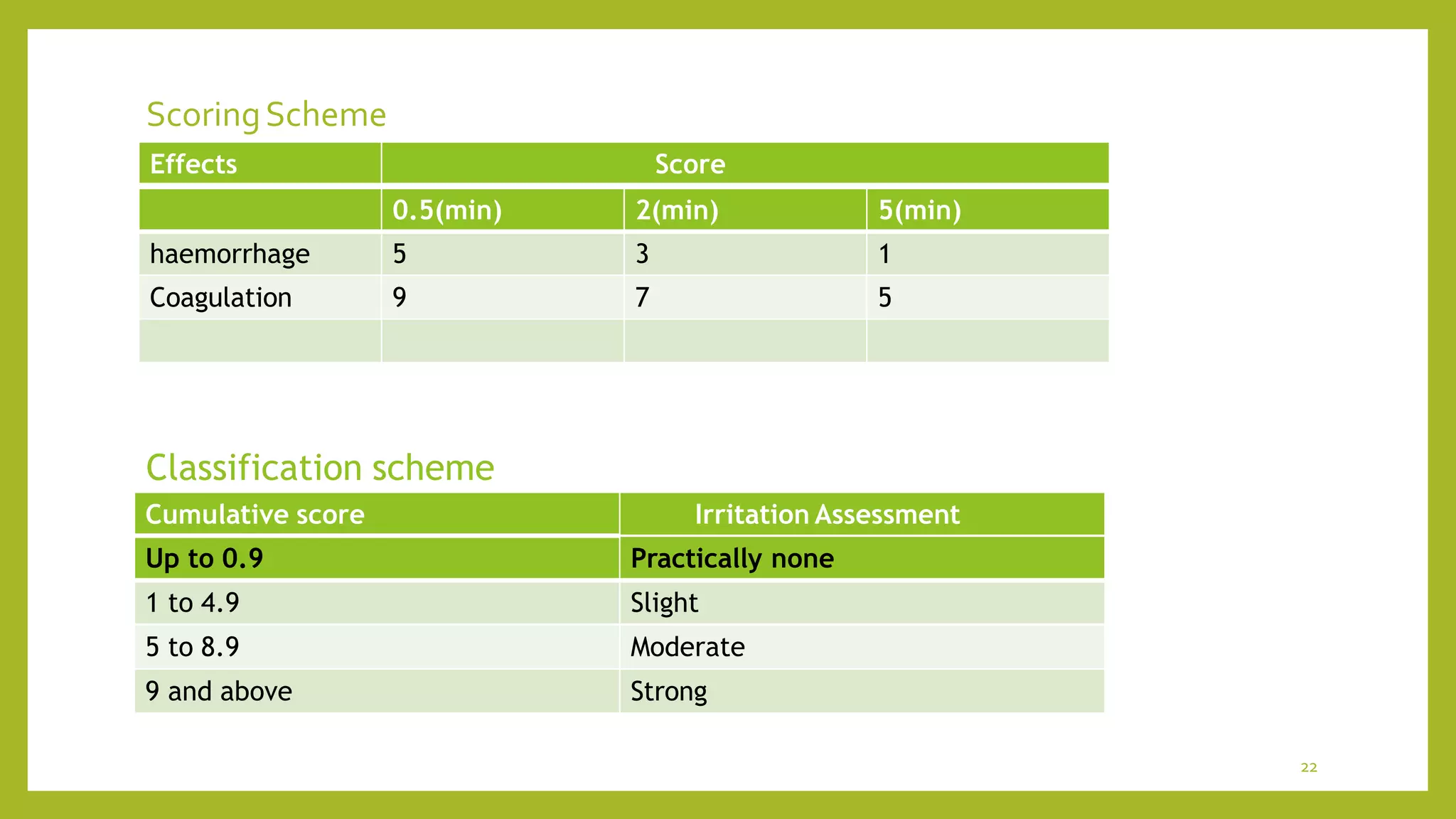
![INVITROMODELS
Instead of using animals, cell and tissue culture can be used to test
product ingredients .
Cell culture are now also used routinely to test substance for mutagenic
properties.
Can be grown as independent cell lines or preserve the
architecture of entire organ as organ culture and tissue culture.
Stem cells are also used as in vitro models.
SOURCE OF TISSUE FOR IN VITRO METHODS
Avian chick embryo
Rodents[rat and mice], wild type, transgenic , embryonic,
post natal , adult
Human cells [neural progenitor cells from aborted foetus and
stem cell lines]](https://image.slidesharecdn.com/alternativemethodstoanimaltoxicitytestingbysanchitdhankhar-210612041919/75/Alternative-methods-to-animal-toxicity-testing-11-2048.jpg)

![INSILICO[COMPUTER MODELLING]
TECHNIQUES
• Without animal dissection computer generated stimulation are
used to predict the various possible biological and toxic effects
of a chemical or potential drug candidate.
VARIOUS TYPES IN SILICO MODELS
Computer aided molecular drug design [CADD]
Quantitative structureactivity relationship
Computer assistedlearning[CAL]
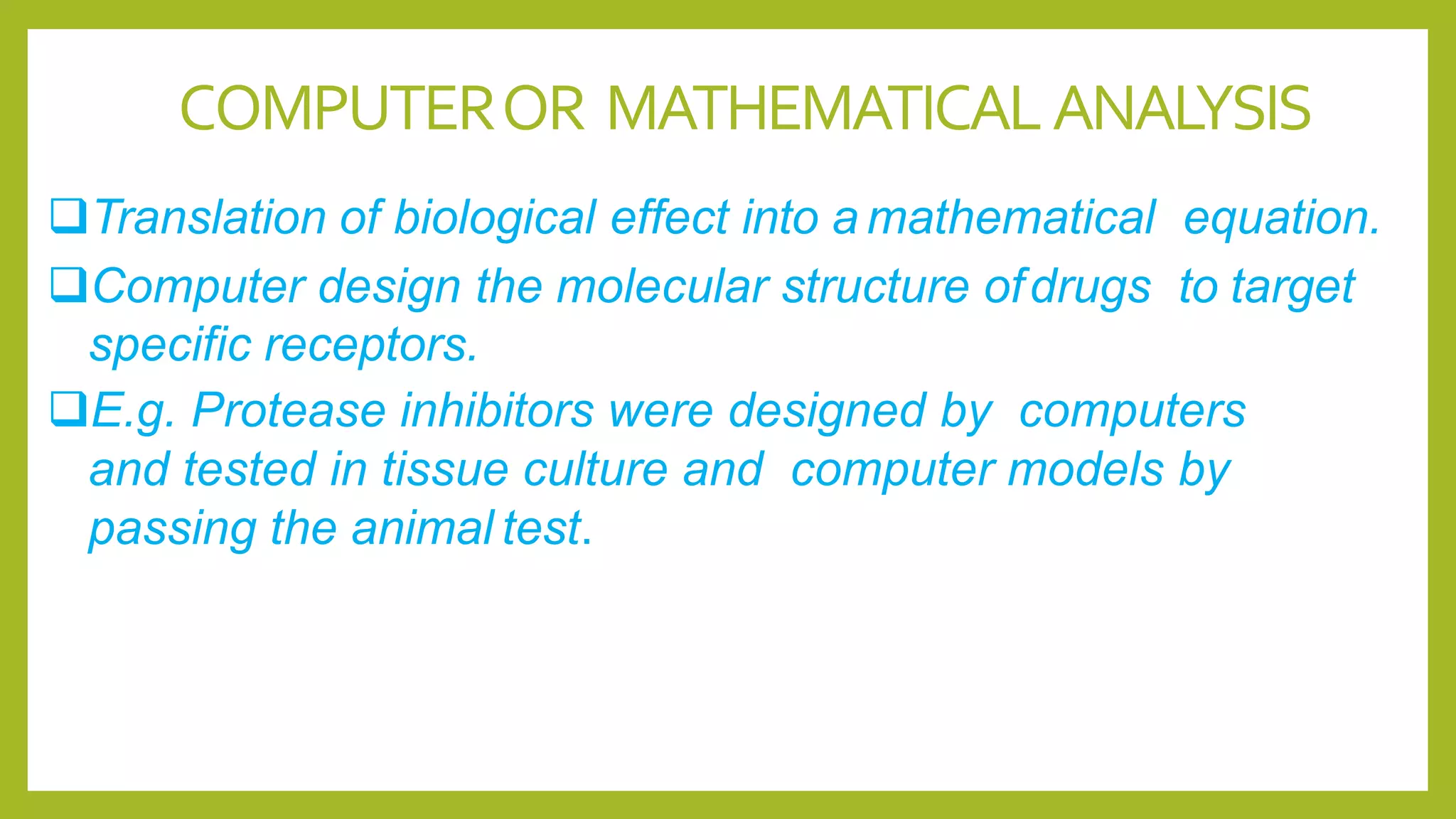
Computer or mathematical analysis
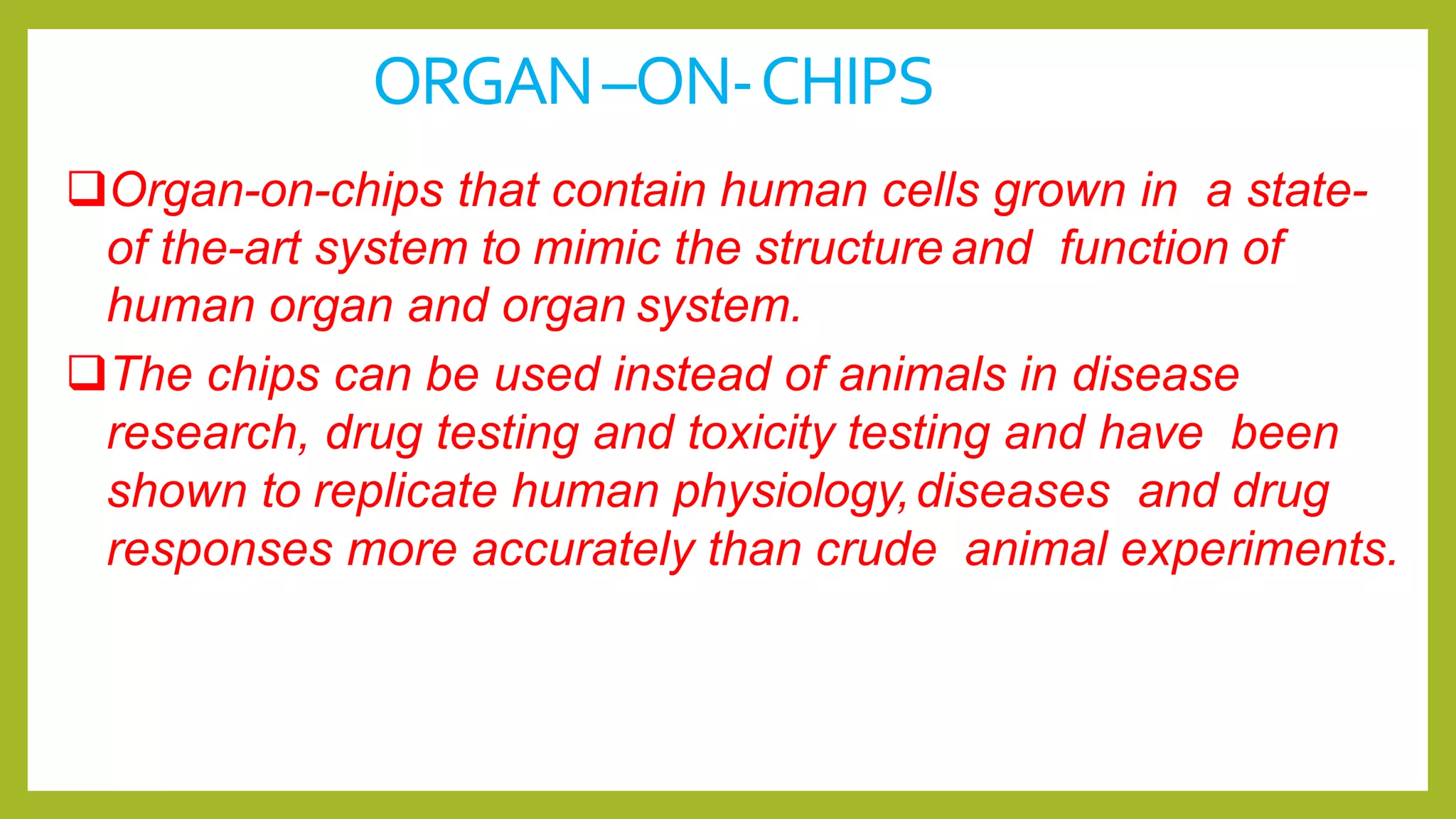
Organ on chips](https://image.slidesharecdn.com/alternativemethodstoanimaltoxicitytestingbysanchitdhankhar-210612041919/75/Alternative-methods-to-animal-toxicity-testing-13-2048.jpg)
![COMPUTERAIDEDDRUG DESIGN[CADD]
It is used to predict the receptor binding site fora potential
drug molecule.
CADD works to identify the probable binding site and
hence avoid testing of unwanted chemicals having no
biological activity.
Computational approach to discover, develop and analyse
drugs.](https://image.slidesharecdn.com/alternativemethodstoanimaltoxicitytestingbysanchitdhankhar-210612041919/75/Alternative-methods-to-animal-toxicity-testing-14-2048.jpg)
![COMPUTERAIDEDDRUG DESIGN[CADD]
It is used to predict the receptor binding site
fora potential drug molecule.
CADD works to identify the probable binding
site and hence avoid testing of unwanted
chemicals having no biological activity.
Computational approach to discover, develop
and analyse drugs.](https://image.slidesharecdn.com/alternativemethodstoanimaltoxicitytestingbysanchitdhankhar-210612041919/75/Alternative-methods-to-animal-toxicity-testing-15-2048.jpg)
![COMPUTERASSISTED LEARNING [CAL]
CAL is an interactive computer assisted
learning program without involvement of real
experimental tools.
The cost is much less than the
traditional laboratory practices.
Two software are currently used inIndia:
Ex- pharm
X- cology](https://image.slidesharecdn.com/alternativemethodstoanimaltoxicitytestingbysanchitdhankhar-210612041919/75/Alternative-methods-to-animal-toxicity-testing-16-2048.jpg)