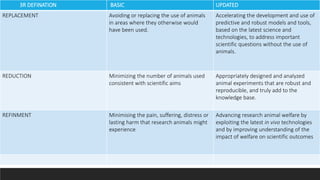
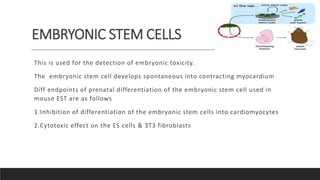
Alternative toxicity methods are being developed to replace animal testing. Some alternatives include in vitro tests using cell and tissue cultures, computer modeling like QSAR, and organ-on-chip microfluidic devices containing human cells. These alternative methods aim to reduce and refine animal use in toxicity testing by providing human-relevant data without using live animals.
![Alternative to
animal toxicity
methods
S U B M I T T E D BY: A N A N YA PA N D E Y
M . P H A R M AC Y [ P H A R M AC O LO GY ]
2 ND S E M](https://image.slidesharecdn.com/alternativetoanimaltoxicittesting-220629100933-9cbd8784/75/Alternative-to-animal-toxicit-testing-pptx-1-2048.jpg)






![ALTERNATIVE NON-ANIMAL TEST
In vitro pyrogen test
Embryonic stem cell test [EST]
HET-CAM Test
In silico Test
CADD
CAL
QSAR
ORGAN ON CHIP](https://image.slidesharecdn.com/alternativetoanimaltoxicittesting-220629100933-9cbd8784/85/Alternative-to-animal-toxicit-testing-pptx-9-320.jpg)





![IN-SILICO METHODS
Without animal dissection computer-generated stimulation is used to predict the various possible
biological and toxic effects of a chemical or potential drug candidate
VARIOUS TYPES OF SILICO MODELS
Computer-aided molecular drug design [CADD]
Quantitative structure-activity relationship
Computer assisted learning[CAL]
Computer or mathematical analysis
Organ on chips](https://image.slidesharecdn.com/alternativetoanimaltoxicittesting-220629100933-9cbd8784/85/Alternative-to-animal-toxicit-testing-pptx-16-320.jpg)
![COMPUTERAIDEDDRUG DESIGN[CADD]
It is used to predict the receptor binding site for a potential drug molecule. CADD works to identify the probable binding site
and hence avoid testing of unwanted chemicals having no biological activity. A computational approach to discovering,
developing, and analyzing drugs.
It is used to predict the receptor binding site for a potential drug molecule.
CADD works to identify the probable binding site and hence avoid testing of unwanted chemicals having no biological
activity.
A computational approach to discovering, developing, and analysing drugs.
COMPUTER-ASSISTED LEARNING [CAL]
CAL is an interactive computer-assisted learning program without the
involvement of real experimental tools.
The cost is much less than the traditional laboratory practices.
Two software are currently used in India: Ex- pharm X- cology
QUANTITATIVE STRUCTURE-ACTIVITY RELATIONSHIP
Computer programs can predict the toxicity of new chemicals or drugs based on their similarity to more established
compounds.
The principle is that similar chemicals should have similar biological properties.
QSAR has been widely used in medicinal chemistry as support in drug discovery and development process as well as in the
study of harmful and poisonous substances in toxicological chemistry.](https://image.slidesharecdn.com/alternativetoanimaltoxicittesting-220629100933-9cbd8784/85/Alternative-to-animal-toxicit-testing-pptx-17-320.jpg)


