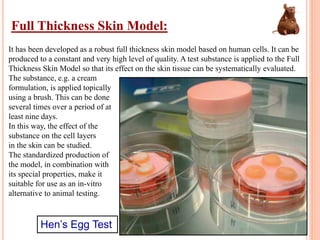
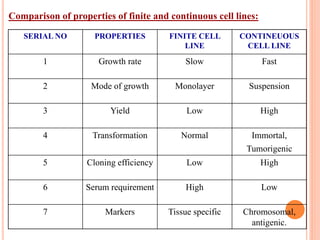
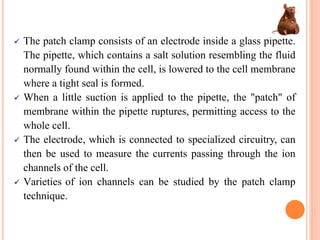
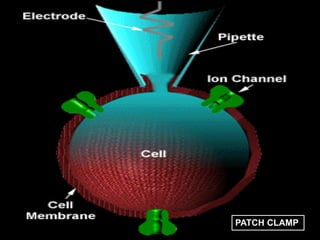
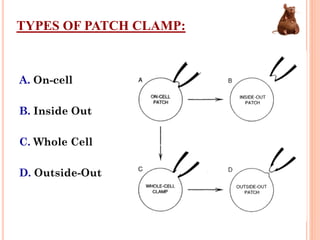
Toxicity studies are generally performed to determine drug effects that cannot be evaluated through standard pharmacology profiles or that only occur with repeated administration. Most toxicity tests are performed in two species, such as a rodent and non-rodent, to avoid overlooking unexpected adverse effects before introducing new chemical entities into humans. While animal testing has faced controversy, alternatives using biotechnology tools such as transgenic animal models, cell cultures, and in silico methods are being developed wherever possible to reduce animal use. These alternative techniques include cell line techniques, full thickness skin models, and patch clamp methods to study ion channels at a cellular level.