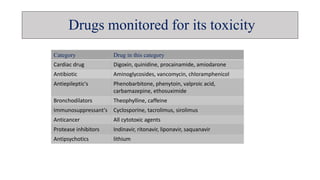
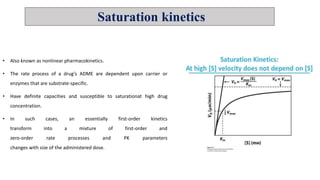
The document discusses toxicokinetics, which involves the study of drug safety evaluation through the generation of pharmacokinetic data across different animal species. It outlines the objectives, principles, and applications of toxicokinetic studies, as well as the importance of saturation kinetics in non-linear pharmacokinetics. Additionally, it emphasizes the need for such studies in drug development and clinical safety assessments.