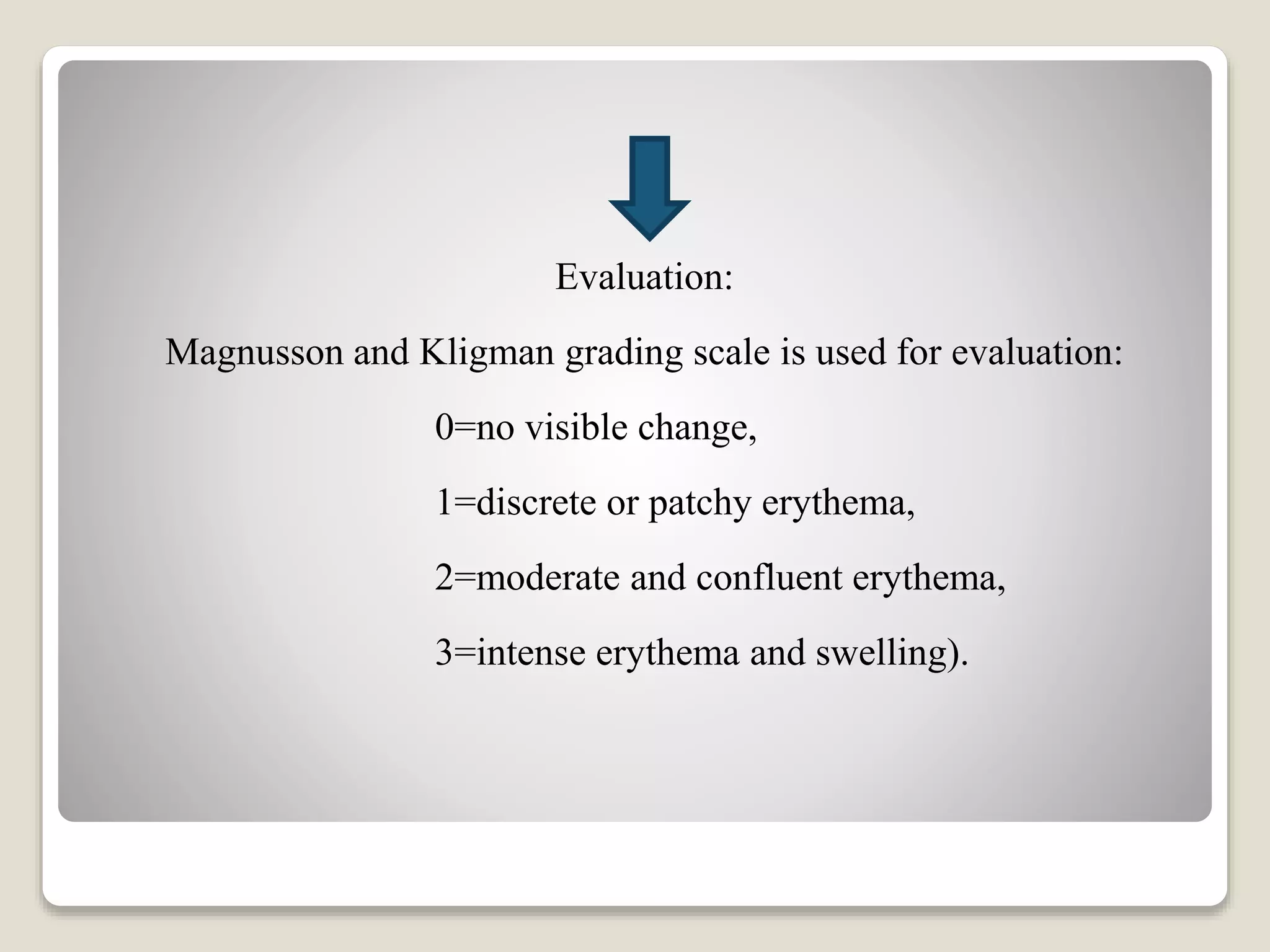
The document discusses pharmacological and toxicological screening methods for acute eye irritation and skin sensitization, highlighting evaluation techniques involving animal testing. For eye irritation, scores of conjunctiva, cornea, and iris lesions are assessed post-exposure, while skin sensitization is defined as an allergic reaction to skin contact and tested through various methods including guinea pig and local lymph node assays. The methods aim to determine both immediate and long-term responses to test substances, emphasizing the need for proper controls and ethical treatment of the test animals.