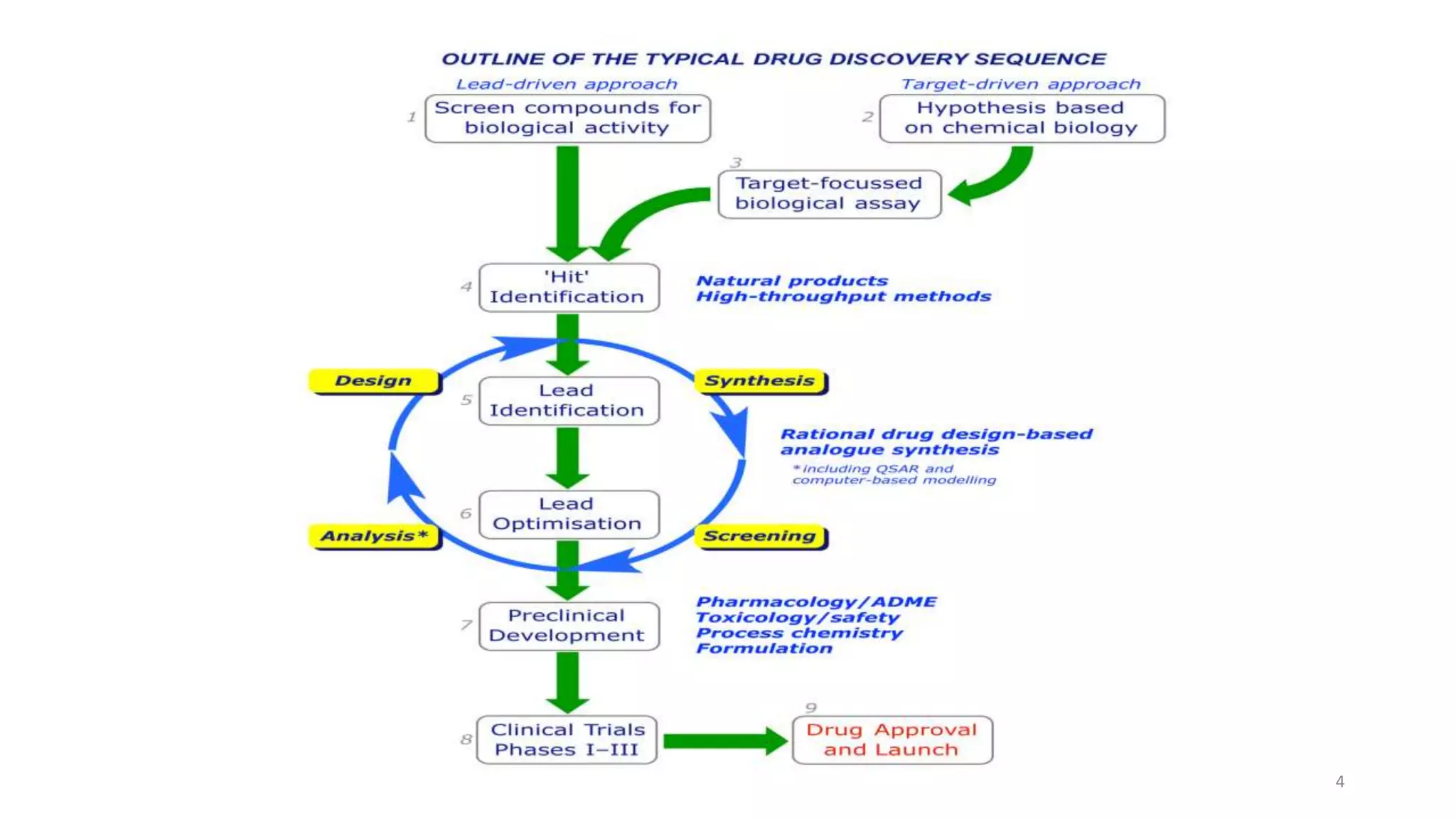
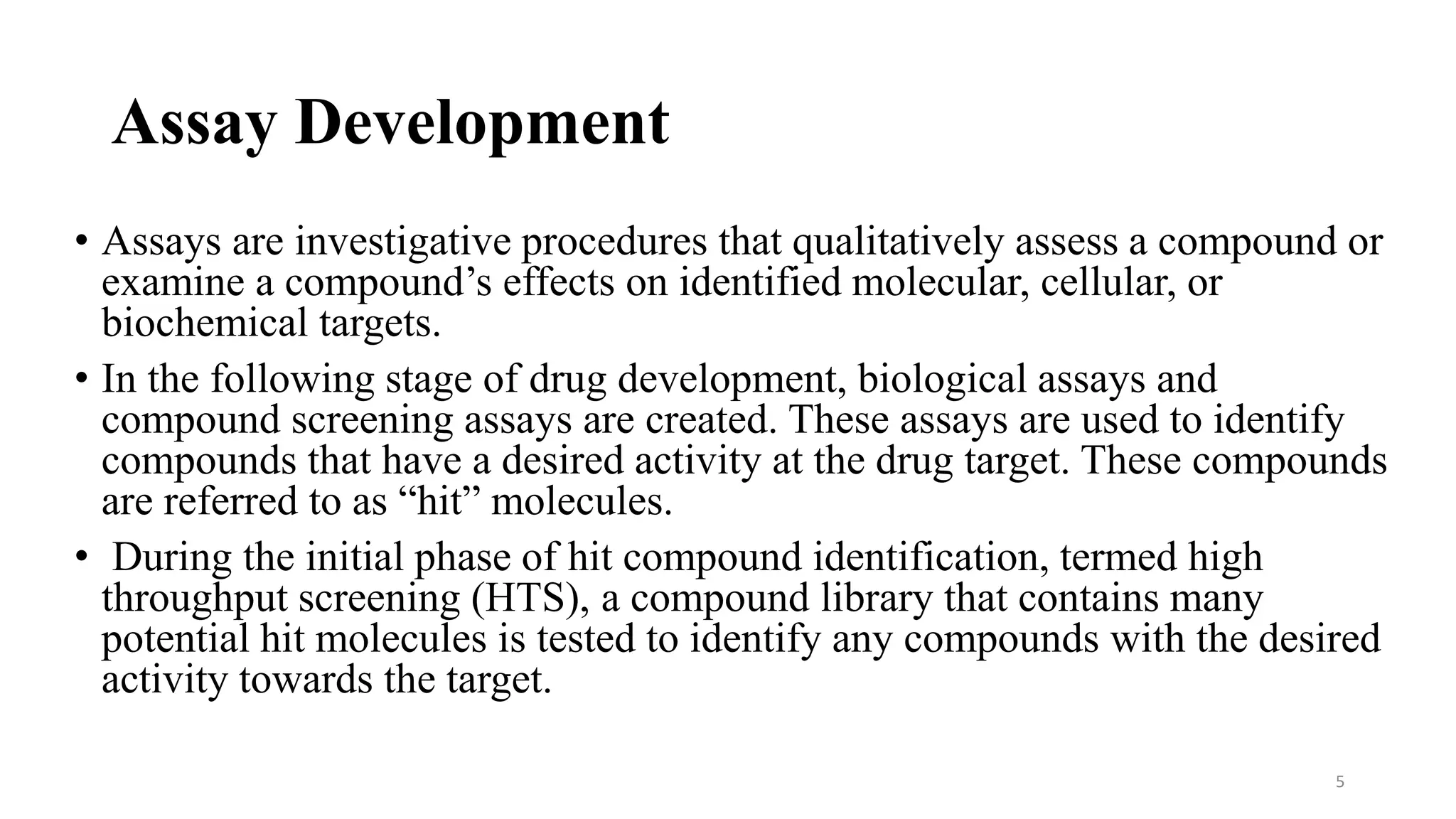
- Assay development is the process of creating biological and compound screening assays to identify compounds, called "hits", that have desired activity at drug targets. This involves developing biochemical and cell-based assays.
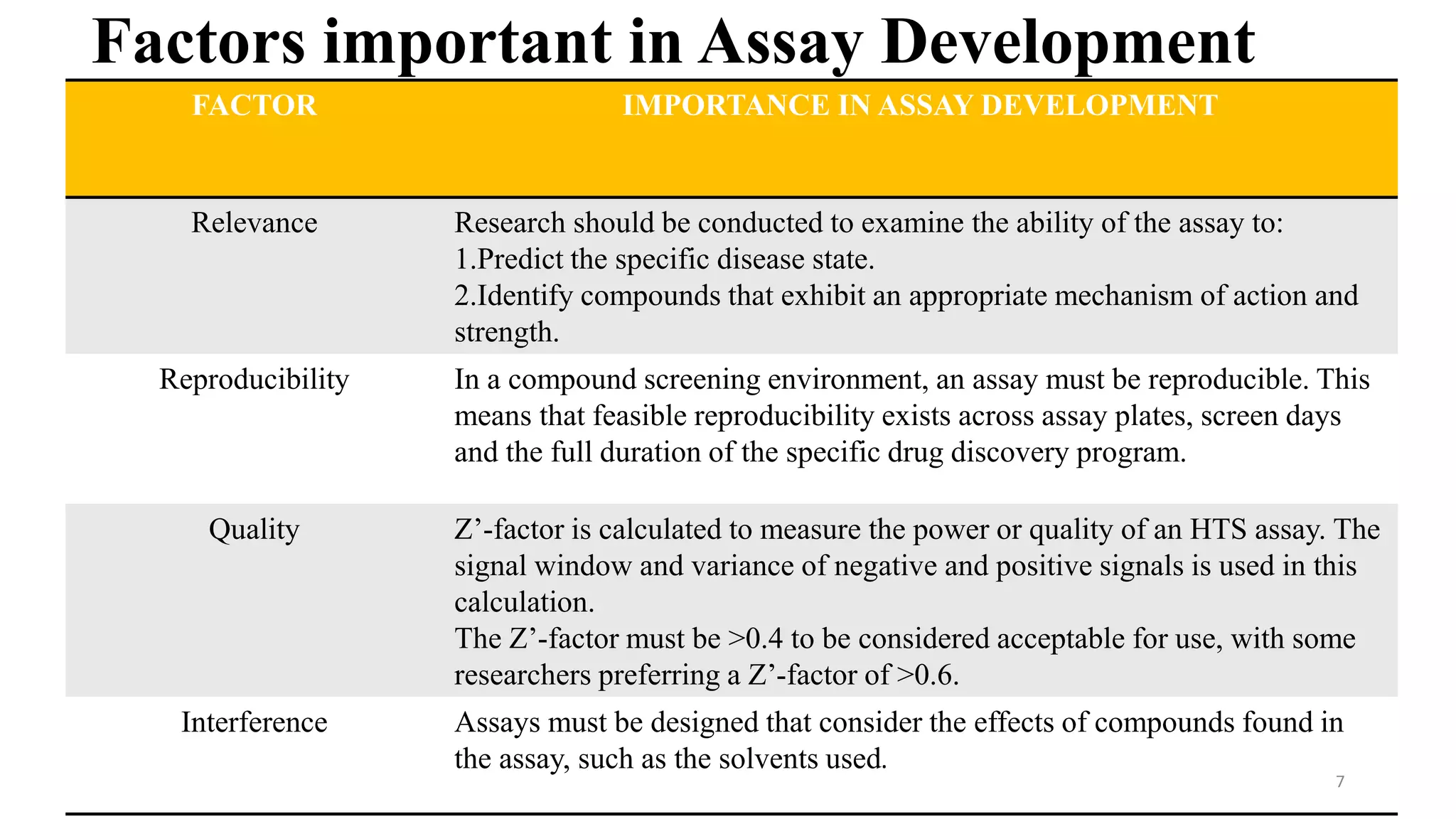
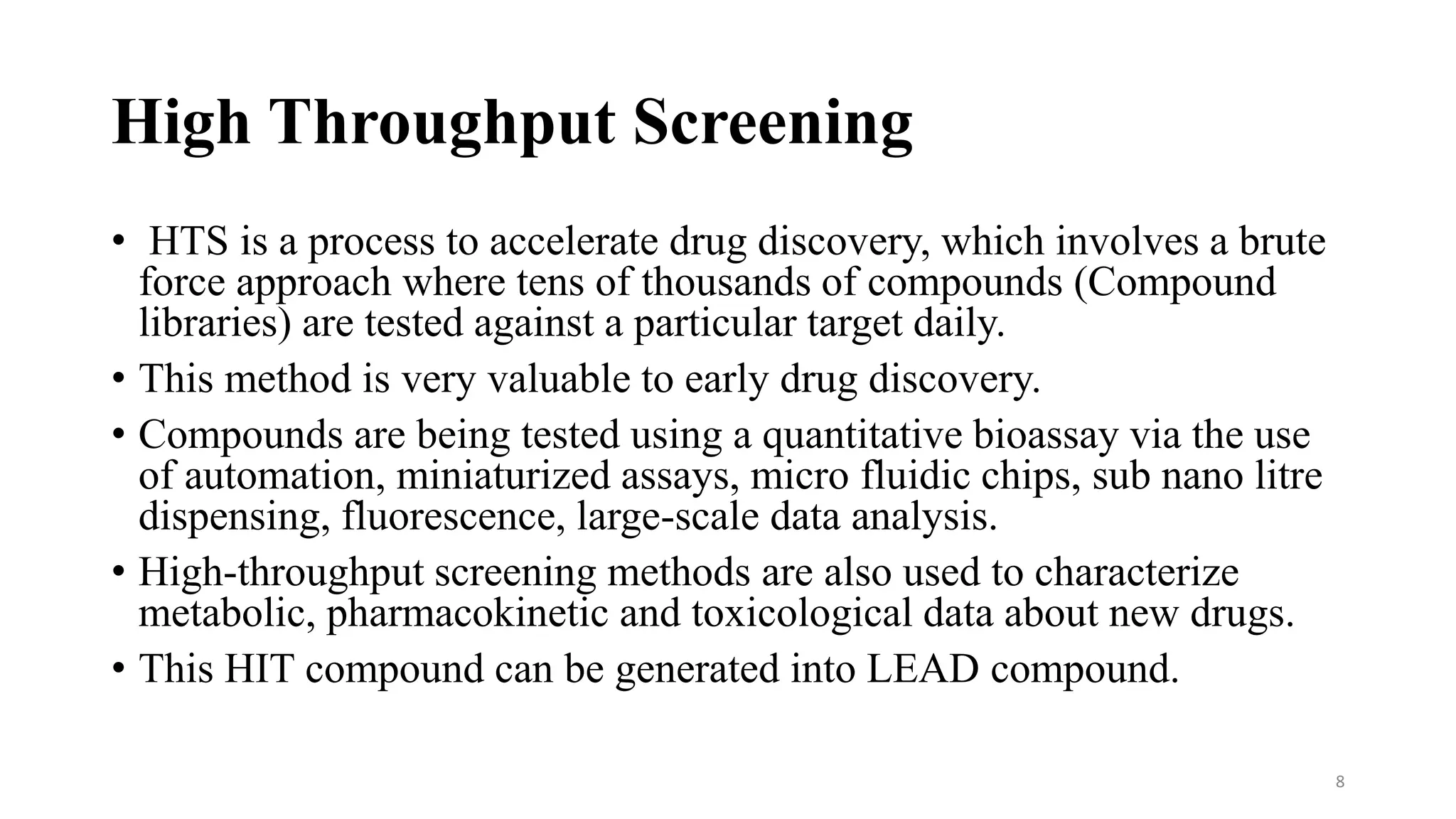
- Key factors in assay development include relevance, reproducibility, quality as measured by Z'-factor, and avoiding interference. High throughput screening uses automation to test tens of thousands of compounds against targets daily using miniaturized assays.
- Biochemical assays use purified protein or enzyme targets, while cell-based assays examine responses at transcriptional, proliferation, or second messenger levels. Automation and robotics are important for achieving desired screening rates in high throughput screening.