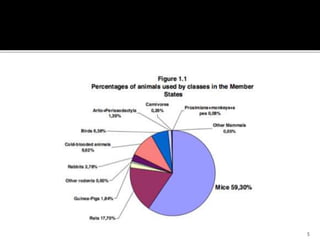
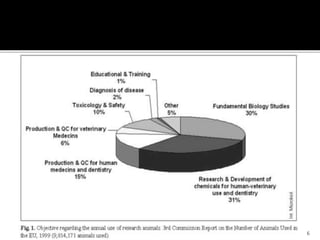
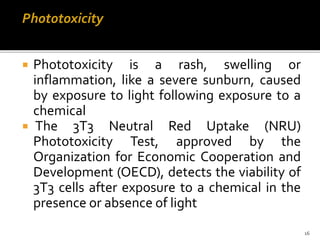
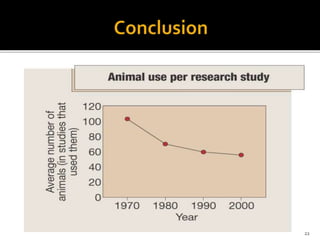
This document discusses concepts of in-vitro screening as an alternative to animal testing. It describes some of the major alternatives like cell culture techniques and computer simulation that aim to replace, reduce or refine animal testing. Specifically, it provides examples of how cell cultures have been developed as alternatives for tasks like monoclonal antibody production and toxicity testing. It also discusses the "Three Rs" framework of replacing, reducing and refining animal use in testing.